Abstract
Near infrared (NIR) spectroscopy is a well-established technique that is widely employed in agriculture, chemometrics, and pharmaceutical engineering. Recently, the technique has shown potential in clinical orthopaedic applications, for example, assisting in the diagnosis of various knee-related diseases (e.g., osteoarthritis) and their pathologies. NIR spectroscopy (NIRS) could be especially useful for determining the integrity and condition of articular cartilage, as the current arthroscopic diagnostics is subjective and unreliable. In this work, we present an extensive dataset of NIRS measurements for evaluating the condition, mechanical properties, structure, and composition of equine articular cartilage. The dataset contains NIRS measurements from 869 different locations across the articular surfaces of five equine fetlock joints. A comprehensive library of reference values for each measurement location is also provided, including results from a mechanical indentation testing, digital densitometry imaging, polarized light microscopy, and Fourier transform infrared spectroscopy. The published data can either be used as a model of human cartilage or to advance equine veterinary research.
Subject terms: Biomedical engineering, Biophysics, Cartilage, Near-infrared spectroscopy
| Design Type(s) | biomechanics data analysis objective • methodology testing objective |
| Measurement Type(s) | cartilage • biomechanical data • histology |
| Technology Type(s) | near-infrared spectroscopy • biomechanical data analysis |
| Factor Type(s) | Region_Of_Interest • Biospecimen • metacarpophalangeal joint |
| Sample Characteristic(s) | Equus • metacarpophalangeal joint |
Machine-accessible metadata file describing the reported data (ISA-Tab format)
Background & Summary
Osteoarthritis (OA) is the most common musculoskeletal disorder1, characterized by the deterioration of articular cartilage and subchondral bone. Articular cartilage is an avascular and aneural connective tissue that covers the ends of bones in articulating joints (e.g., the knee). The main role of articular cartilage is to enable near-frictionless movement between the bones and to distribute mechanical loads. OA can be triggered by prolonged abnormal joint loading (for example, being overweight), whereas post-traumatic OA (PTOA) can be caused by sudden physical trauma, such as a fall or a slip2–5. Once triggered, the OA progresses due to the poor self-repair capabilities of articular cartilage. Currently, no cure exists for OA; however, the progression of the disease can be slowed down with medication and conventional physical therapy. Advanced OA is typically very painful and can eventually require joint replacement surgery6. Furthermore, OA-related medical costs and disabilities make it a substantial socio-economic burden. Since cartilage is an aneural tissue, early-stage OA can be asymptomatic and, therefore, difficult to detect. In order to effectively plan treatment and improve the outcomes of OA patients, early and accurate diagnosis of cartilage defects is vitally important.
Initial diagnosis of OA is usually performed by a medical doctor through physical examination. Modern medical imaging techniques are effective for verifying the initial detection of OA. However, during joint repair procedures, surgeons rely on traditional arthroscopic tools when evaluating cartilage condition. These tools are currently limited to an endoscopic camera (for visual observation) and a metal hook (for manual palpation of the tissue)7. While these methods are considered to be the gold standard, they are highly subjective with poor repeatability8–10. Quantitative techniques for real-time diagnostics of cartilage could substantially improve the decision-making ability of orthopaedic surgeons during these repair procedures11–13.
Near infrared spectroscopy (NIRS) has demonstrated potential as a diagnostic tool for evaluating cartilage condition12,14,15. NIRS is a non-destructive optical sensing technique in which the absorbance of a target sample at different wavelengths within the near infrared (NIR) spectral range is measured. Changes in the composition and structure of cartilage can be observed in the measured spectrum. As OA-related degeneration of articular cartilage induces changes in both the chemical composition and structure, NIRS can indirectly quantify the condition of the tissue.
NIRS-based arthroscopy requires sophisticated multivariate statistical models (such as, partial least squares regression, principal component regression, neural networks, etc.) that relate the measured NIR spectra to the various properties of cartilage. To develop any of these techniques, large data library from human cadavers or suitable animal models (i.e., large mammals) is often required. Ideally, these datasets should consist of multiple cartilage samples with varying degrees of tissue defects. In addition to NIRS measurements, a set of reference variables (i.e., different biomechanical and chemical properties that characterize the tissue) should be included. To facilitate the development of better NIRS models for cartilage evaluation, we are publishing this dataset collected from equine fetlock joints.
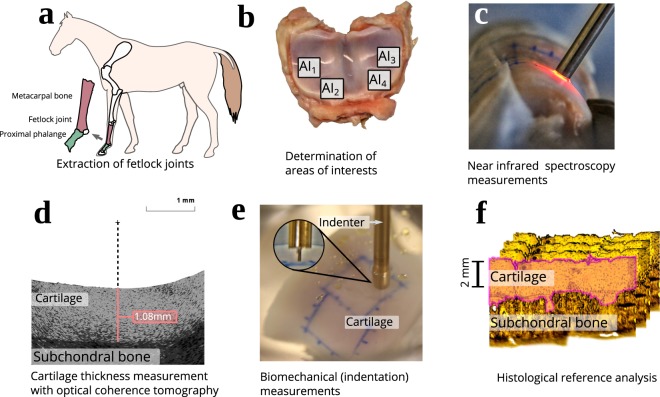
This dataset was collected from five mature equine fetlock joints (Fig. 1a) obtained from a slaughterhouse in Utrecht. Representative areas of interest (AI) with varying degrees of cartilage defects were selected and graded by two experienced veterinary surgeons (Fig. 1b). These regions were first measured with NIRS in a grid-like pattern (Figs 1c and 2). Corresponding reference values for each site were then measured using optical coherence tomography (OCT, Fig. 1d), a biomechanical testing protocol (Fig. 1e), and a battery of histological methods (Fig. 1f). The biomechanical protocol was employed to determine various mechanical properties of the tissue through indentation testing. OCT was used to determine the thickness of the non-calcified cartilage layer at each measurement site. Histological methods (polarized light microscopy (PLM), digital densitometry (DD) of Safranin-O stained samples, and Fourier-transform infrared microspectroscopy (FTIR)) were used as a post-hoc step to determine cartilage collagen fibre orientation, proteoglycan content, and collagen content, respectively, from a selected subset of measurement points. Histological analysis was performed on thin slices cut from the original samples. The collected data was first reported in the studies of Sarin et al.16,17 and has since been utilized in Prakash et al.18,19.
Fig. 1.
The measurement workflow of the samples. (a) Anatomical location of the fetlock joint. (b) An example of areas of interest. (c) Near infrared spectroscopic measurement. (d) Determination of cartilage thickness using optical coherence tomography. (e) Material testing setup for conducting the biomechanical indentation tests. (f) An example histological section of equine cartilage and subchondral bone.
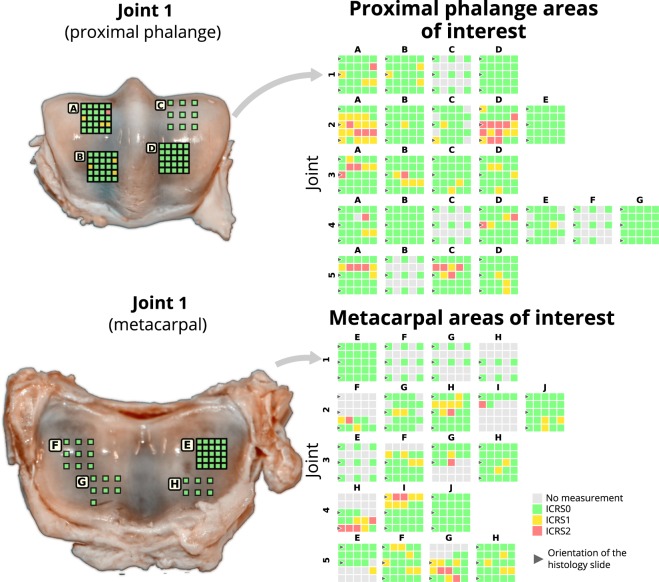
Fig. 2.
All areas of interest (AI) and associated measurement points with their evaluated cartilage condition (according to ICRS grade scored from optical coherence tomography images)17. The locations and size of the AIs are demonstrated on the proximal phalange and metacarpal surfaces of joint 1.
We believe the published data can be useful for equine veterinary research or as an animal model of human cartilage research. The provided NIR spectra, in combination with biomechanical indentation testing, can be used to train models capable of predicting various biomechanical properties of cartilage. Likewise, the combination of NIRS with the histological reference parameters can be utilized to predict properties related to the composition and structure of the tissue. The development of new calibration techniques for NIRS is an active field of research and open datasets are used to evaluate the performance of these techniques. In comparison to publicly available datasets20–27, the presented data comprises a high number of samples, a large selection of reference variables, and represents various tissue conditions. Currently available NIRS datasets rarely contain measurements of biological tissue but rather focus on agricultural20–22, chemical25 or food products26,27.
While the main focus of the dataset is in the development of NIRS techniques for evaluating cartilage condition, the broad library of reference variables can also be used to study the structure-function relationship of articular cartilage. These functional, compositional, and structural properties could be utilized, for instance, in simulation studies of joint physiology28 or simply as a reference library. Finally, the NIRS measurements combined with the structural and compositional properties of cartilage could, for instance, be used to model the interaction between NIR light and articular cartilage in order to gain a better understanding of the sensitivity of the NIRS technique as a function of penetration depth.
Methods
The following sections (i.e., Sample extraction, Near infrared spectroscopy, Measurement of cartilage thickness, Biomechanical testing, and Histology), describing the methods utilized in this study, are expanded versions of descriptions in our related works16,17. The employed measurement techniques and the corresponding data are summarized in Table 1.
Table 1.
Summary of the measurement techniques employed, and corresponding data collected.
| Technique | Measurement locations | Data |
|---|---|---|
| Near infrared spectroscopy | 869 | Absorption spectra (λ = 700 nm–1150 nm) with 3 repetitions per measurement location |
| Optical coherence tomography | 869 | Cartilage and calcified cartilage thickness |
| Biomechanics (indentation) | 1st protocol, 869 | Raw signals (time, location, and load) and calculated parameter: instantaneous modulus |
| 2nd protocol, 202 | Raw signals (time, location, and load) and parameters: equilibrium and dynamic moduli | |
| Digital densitometry | 530 | Depth-wise optical density profile (average of three histological sections) |
| Polarized light microscopy | 530 | Depth-wise collagen orientation and retardance profiles (average of three histological sections) |
| Fourier transform infrared microspectroscopy | 530 | Depth-wise proteoglycan and amide profiles (one histological section) |
Sample extraction
Metacarpophalangeal joints (N = 5) were extracted from mature equines which were obtained from a slaughterhouse in Utrecht (Equine Slaughterhouse Van de Veen, Nijkerk, Netherlands); no ethical permission was required. A total of 44 AIs (dimensions 15 × 15 mm) with varying cartilage condition were selected from the joints by two experienced equine surgeons. Approximately half of the AIs were selected from the articular surface of the metacarpal bone and the other half from the surface of the proximal phalanx. Each AI was independently scored by the two surgeons according to the International Cartilage Repair Society (ICRS) scoring system. ICRS scores were used to divide the AIs into healthy (N = 19) and damaged (N = 25) categories. Each individual AI was further subdivided into a uniform 5 × 5 grid (25 measurement points) where NIRS and reference measurements were conducted. In total, 869 measurement points from all AIs were subjected to further analysis, while the remaining 231 points were excluded due to fully eroded cartilage or due to limitations imposed by extensive biomechanical measurements (sample preservation).
Near infrared spectroscopy
NIRS measurements were performed using a system consisting of a halogen light source (wavelength 360–2500 nm, power 5 W, optical power 239 µW (in 600 µm fibre), Avantes BV, Apeldoorn, Netherlands), a spectrometer (wavelength 200–1160 nm, AvaSpec-ULS2048XL, Avantes BV), and a diffuse reflectance fibre optic probe16,17. The probe (d = 5 mm) consists of seven fibres (dfibre = 600 µm) within the central window (d = 2 mm), of which the central fibre was utilized for collecting diffuse reflected light. Data acquisition was performed with Avasoft 8.0 software (Avantes BV). Dark and reference spectra were acquired from non-reflectance (black rubber pad) and reflectance standards (Spectralon, SRS-99, Labsphere Inc., North Sutton, USA), respectively, with the fibre optic probe in perpendicular contact during measurement in order to minimize environmental factors such as stray light. The absorbance at each wavelength (Aλ) was determined as follows:
where Sλ is sample spectrum, Dλ is the dark spectrum, and Rλ is the reference spectrum. The absorption spectrum for each measurement location was determined as the average of three consecutive spectral measurements, with each spectrum consisting of eight coadded acquisitions. Data within the spectral region of 700–1050 nm was utilized (Fig. 3a) since light in the visible region penetrates deeper into the tissue and includes strong contributions from the underlying subchondral bone29,12. Physiological condition of articular cartilage was preserved by constantly spraying phosphate-buffered saline (PBS) on the sample surface and placing PBS soaked gauze on cartilage surrounding the measurement points. After NIRS, the samples were immersed in PBS and stored at −20 °C until required for reference analyses.
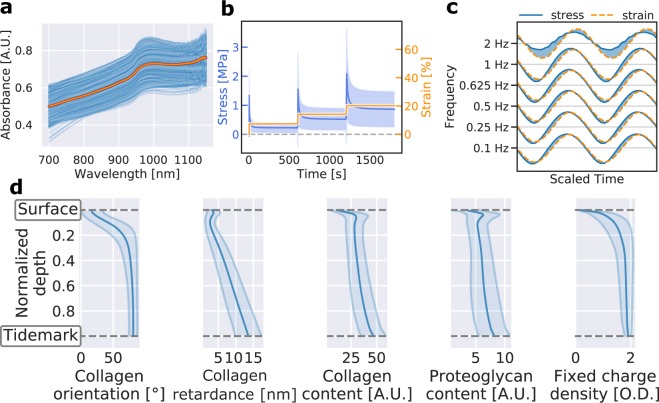
Fig. 3.
Visualization and technical validation of acquired data. (a) All NIR spectra. The thick red line represents the average spectrum. (b) Average stress-relaxation curves of cartilage samples. The shaded region corresponds to the standard deviation of stress-values. (c) Average stress-strain behaviour per testing frequency in dynamic mechanical testing. Shaded region illustrates the phase difference between stress and strain. (d) Averaged depth-wise profiles for collagen network orientation (PLM), retardance (PLM), and content (FTIR) as well as proteoglycan content (FTIR) and fixed charge density (DD). Shaded regions represent the standard deviation.
Since spectral data are likely to include hardware-related noise, spectral preprocessing is required to eliminate noise without degrading essential information. The NIR spectra included in this dataset has not been preprocessed in any way, allowing the user to freely choose preprocessing methods they deem necessary. In the original studies of Sarin et al., a third-degree Savitzky-Golay filter was utilized for preprocessing prior to analysis. The second derivative spectra were also calculated to remove baseline offset and the dominant linear term from the spectral data30. This preprocessing technique was selected as it enhances identification of small and subtle absorption peaks which are not easily resolved visually in the original spectrum30,31. Additionally, normalization techniques, such as multiplicative scatter correction and standard normal variate, can be employed to further enhance spectral changes. We have provided an example MATLAB script of a typical analysis which also includes spectral preprocessing (see “Data Records” section).
Measurement of cartilage thickness
Samples were thawed in PBS at room temperature and subjected to OCT (wavelength 1305 ± 55 nm, axial resolution <20 µm, lateral resolution 25–60 µm; Ilumien PCI Optimization System, St. Jude Medical, St. Paul, MN, USA) to determine non-calcified cartilage thickness without damaging the cartilage (Fig. 1d)16,17. The average thickness of equine cartilage was 0.89 mm with a range between 0.32 and 1.82 mm. This information was later required in biomechanical measurements. OCT images were also utilized in the ICRS scoring of cartilage condition17.
Biomechanical testing
The bone end of each sample was glued on a custom-made sample holder which was mounted on a goniometer (#55–841, Edmund Optics Inc., Barrington, NJ, USA)16,17. The sample was fully immersed in PBS supplemented with Antibiotic-Antimycotic solution (A5955, Sigma-Aldrich) during measurements (Fig. 1e).
Cartilage biomechanical properties were determined through indentation testing with a custom material tester using plane-ended cylindrical indenters (d = 0.53 mm & 0.51 mm). The material tester consisted of a load cell (5 mN resolution, Sensotec, Columbus, OH, USA) and an actuator with a displacement resolution of 0.1 µm (PM500–1 A, Newport, Irvine, CA, USA). Cartilage surface and the indenter were aligned perpendicular, followed by driving the indenter into contact with the surface (pre-stress = 12.5 kPa)14. Contact was ensured by indenting the specimen 2% of its thickness five times.
To ensure sample preservation during the extensive biomechanical measurements, two different testing protocols (protocols 1 and 2, see Fig. 3b,c) were used. First, protocol 1, consisting of a single 7.5% strain step indentation at a strain rate of 100%/s, was performed for all measurement points. Second, a more extensive protocol 2 was performed on a select set of measurement locations (five measurement points per AI, N = 202). Protocol 2 consisted of an indentation test with three cumulative 7.5% strain steps with 10-minute relaxation time between steps (strain rate 100%/s) followed by four cycles of dynamic sinusoidal loading at frequencies 0.1, 0.25, 0.5, 0.625, 0.833, 1.0, and 2.0 Hz (amplitude of 2% of the remaining cartilage thickness).
Equilibrium, dynamic, and instantaneous moduli were calculated with solution derived from Hayes et al.32 with Poisson’s ratios of 0.1, 0.5, and 0.5, respectively33. Equilibrium modulus was determined from the linear slope of the equilibrium stress-strain curve, whereas dynamic modulus was calculated from sinusoidal loading as the ratio of stress and strain amplitudes. Instantaneous modulus was determined from the first step of the stress-relaxation curves of both protocols
Histology
Osteochondral samples were processed for histology by extracting the measurement locations (Fig. 2, black arrows), followed by fixing in formalin, decalcification in EDTA, and embedding in paraffin blocks16,17,34–36. Sections (N = 7) were cut with a microtome for the histological imaging modalities, i.e., FTIR microspectroscopy (N = 1), PLM (N = 3), and DD (N = 3). The section thicknesses for the imaging modalities were 5 μm, 5 μm, and 3 μm, respectively.
FTIR microspectroscopy was utilized to determine collagen and proteoglycan distributions from the histological sections by mapping 500-μm-wide areas covering the full cartilage thickness in the mid infrared (MIR) region. Similar regions were imaged with PLM and DD. FTIR measurements were conducted with a Thermo iN10 FT-IR microscope (Thermo Nicolet Corporation, Madison, WI, USA) in transmission mode at a spectral resolution of 4 cm−1 and pixel size of 25 × 25 μm2. Four repetitive measurements per pixel were acquired and averaged. The collagen and proteoglycan contents were determined as the integrated area of the amide I peak (1584–1720 cm−1) and the carbohydrate region (984–1140 cm−1), respectively37.
PLM enabled determination of collagen fibre orientation and birefringence of the cartilage samples. PLM imaging was conducted using an Abrio PLM system (CRi, Inc., Woburn, MA, USA) mounted on a conventional light microscope (Nikon Diaphot TMD, Nikon, Inc., Shinagawa, Tokyo, Japan). The Abrio system consists of a green bandpass filter, a circular polarizer, and a computer-controlled analyser composed of two liquid crystal polarizers and a CCD camera. All specimens were imaged at identical orientation with a 4.0x objective, which resulted in a pixel size of 2.53 × 2.53 μm2. In the orientation images, 0 degrees corresponds to the orientation parallel to cartilage surface and 90 degrees perpendicular to cartilage surface.
For DD measurements, the 3 µm thick sections were stained with Safranin-O to determine proteoglycan distribution26. The system consists of a light microscope (Nikon Microphot-FXA, Nikon Co., Tokyo, Japan), equipped with a light source, a monochromatic filter, and a 12-bit CCD camera (ORCA-ER, Hamamatsu Photonics K.K., Hamamatsu, Japan). The system was calibrated with neutral density filters (Schott, Mainz, Germany) covering optical density (OD) range from 0 to 3.0. The samples were imaged with a 4.0x objective resulting in a pixel size of 1.56 × 1.56 μm2.
Data Records
The data records consist of four MATLAB (MathWorks Inc., Natick, MA, USA) .mat files housed within figshare38. The nirs_and_references.mat within figshare38 contains all of the measured NIR spectra and the associated reference values calculated from the biomechanical tests and the histological analysis (see corresponding Methods-sections for details on how these values were obtained). This dataset is the most important and practical dataset as it combines the measured signal (i.e., NIR spectra) and a set of cartilage properties (such as, cartilage thickness, equilibrium modulus, collagen content, etc.). During the calculation of the reference values, several assumptions were made about the data, influencing the final values. For the sake of completeness, transparency, and better replicability, the original data from the reference measurements (with the exception of OCT and PLM) are also included38. The ftir_raw.mat within figshare38 contains the raw FTIR matrices that were collected from the histological sections as described in the chapter Histology. This data was used for determining the proteoglycan and collagen contents as a function of cartilage depth. The biomech_raw_protocol_1.mat and biomech_raw_protocol_2.mat within figshare38 contain the measured force and displacement data measured using the biomechanical indentation testing protocols (see Biomechanical Testing for details). Each of the .mat files contains a “sample_id” variable, which can be used to link measurements of the same location from different modalities. The motivation for providing raw data was to enable recalculation of the reference variables.
The nirs_and_references.mat contains the NIR spectra and values of the reference parameters which are stored as a MATLAB structure. Each element of the dataset structure corresponds to one measurement point and different fields contain the data. Meta-data, including the joint bone type and AI for each measurement point, is also included. A full list of all the different variables is given in Online-only Table 1: List of variables contained in nirs_and_references.mat.
Online-only Table 1.
List of variables contained in nirs_and_references.mat.
| Variable name | Type | Unit | Description |
|---|---|---|---|
| plm_retardance_profile | vector | nm | PLM retardance profile as a function of cartilage depth. |
| plm_orientation profile | vector | deg. | PLM profile as a function of cartilage depth. |
| ftir_proteoglycan_profile | vector | A.U. | FTIR proteoglycan content profile as a function of cartilage depth. |
| ftir_collagen_profile | vector | A.U. | FTIR collagen content profile as a function of cartilage depth. |
| dd_profile | vector | O.D. | Depth-wise DD profile of the cartilage cross-section. |
| dd_average_surface | scalar | O.D. | Average DD value in superficial cartilage. |
| dd_average_deep | scalar | O.D. | Average DD value in deep cartilage. |
| plm_average_surface | scalar | deg. | Average PLM value in superficial cartilage. |
| plm_average_deep | scalar | deg. | Average PLM value in deep cartilage. |
| ftir_collagen_average_surface | scalar | A.U. | Average collagen content in superficial cartilage (computed from FTIR). |
| ftir_collagen_average_deep | scalar | A.U. | Average collagen content in deep cartilage (computed from FTIR). |
| ftir_proteoglycan_average_surface | scalar | A.U. | Average proteoglycan content in superficial cartilage (computed from FTIR). |
| ftir_proteoglycan_average_deep | scalar | A.U. | Average proteoglycan content in deep cartilage (computed from FTIR). |
| ftir_collagen_2d | matrix | A.U. | 2D cross-section FTIR matrix of collagen content (width x depth) |
| ftir_proteoglycan_2d | matrix | A.U. | 2D cross-section FTIR matrix of proteoglycan content (width x depth) |
| wavelength | vector | nm | Wavelength vector of the NIR spectra. |
| sample_id | string | - | Unique identifier string for this measurement. |
| joint_id | integer | - | Identifier of the fetlock joint. |
| area_of_interest_id | string | - | Identifier for the area of interest. |
| measurement_point_id | integer | - | Identifier for the measurement point inside area of interest. |
| bone_type | string | - | Type of bone surface (metacarpal or proximal phalanx) |
| raw_spectra | matrix | A.U. | Matrix containing three NIR spectra measured from this location. |
| cartilage_thickness | scalar | mm | Thickness of the articular cartilage layer. |
| calcified_layer_thickness | scalar | mm | Cartilage thickness including calcified layer. |
| indenter_diameter | scalar | mm | Diameter of the indenter used for mechanical testing. |
| instantaneous_modulus | scalar | MPa | Value of cartilage instantaneous modulus. |
| equilibrium_modulus | scalar | MPa | Value of cartilage equilibrium modulus. |
| dynamic_modulus_at_01 hz | scalar | MPa | Value of cartilage dynamic modulus at 0.1 Hz loading. |
| dynamic_modulus_at_025 hz | scalar | MPa | Value of cartilage dynamic modulus at 0.25 Hz loading. |
| dynamic_modulus_at_05 hz | scalar | MPa | Value of cartilage dynamic modulus at 0.5 Hz loading. |
| dynamic_modulus_at_0625hz | scalar | MPa | Value of cartilage dynamic modulus at 0.625 Hz loading. |
| dynamic_modulus_at_0833 hz | scalar | MPa | Value of cartilage dynamic modulus at 0.833 Hz loading. |
| dynamic_modulus_at_1 hz | scalar | MPa | Value of cartilage dynamic modulus at 1 Hz loading. |
| dynamic_modulus_at_2 hz | scalar | MPa | Value of cartilage dynamic modulus at 2 Hz loading. |
The ftir_raw.mat contains the raw data matrices of the FTIR microspectroscopy measurements which are also stored as a MATLAB structure. The structure of the dataset is similar to nirs_and_references.mat, where each element of the structure represents a different measurement location. Information about the specific joint and measurement location is encoded in the “sample_id” variable. Variables “wave” and “data” contain the wavenumber vector and the FTIR matrix of that specific point. The FTIR measurement was used to calculate the histological reference values related to proteoglycan and collagen contents.
The biomech_raw_protocol_1.mat contains the raw data from the first biomechanical testing protocol. Information about the measurement location can be found under the “sample_id” variable. Raw data of the indentation testing is stored in the “data” variable and contains the timestamp, position and load of the indenter. The column names of the data-matrix are also stored in the “data_columns” variable. The variable “header” contains measurement-specific information about the test setup.
The biomech_raw_protocol_2.mat contains the raw data from the second biomechanical testing protocol. Each element of the structure corresponds to a biomechanical test conducted at a specific measurement point at a given testing frequency. Measurement point information is stored in the “sample_id” variable and variables “header”, “data”, and “data_columns” are the same as in the biomech_raw_protocol_1.mat. The variable “frequency_hd” corresponds to the frequency at which the dynamic indentation testing was conducted.
Technical Validation
Dataset size (N = 869 or N = 530) is sufficient for constructing and validating multivariate models, e.g., NIRS models. The optimal size of a dataset required to train a multivariate model depends on the application but the general consensus suggests 100 samples as the lower limit39.
More importantly, the spread of data should cover the entire natural range of variation found in the mechanical properties of equine cartilage. An earlier investigation of equine proximal phalanx cartilage (N = 30) by Brommer et al.40 reported thickness values of 0.76 ± 0.13 mm, 0.79 ± 0.05 mm, 0.75 ± 0.10 mm, and 0.78 ± 0.11 mm (multiple values reflect various anatomical locations with varying levels of cartilage degeneration). Corresponding values reported for equilibrium modulus were 1.6 ± 0.6 MPa, 1.0 ± 0.4 MPa, 2.8 ± 1.2 MPa, and 2.2 ± 1.1 MPa. By comparing the values for proximal phalanx in this dataset (thickness = 0.84 ± 0.24 mm and equilibrium modulus = 1.98 ± 1.52 MPa), the biomechanical properties are observed to adequately cover the range of values previously reported for this tissue type although the values are acquired from only five individual joints.
Utilization of this dataset for human studies should be reviewed on a case-by-case basis depending on the application of the data. Generally, large mammals, such as equine and bovine, have been considered as suitable animal models for representing human joint physiology due to similarities in loading, gait and cartilage thickness41. For equine orthopaedics, the dataset can be directly applied as, for example, racehorses often undergo arthroscopic examinations.
To ensure reproducible NIR measurements, each location was measured three times with the coefficient of variation (CV) of the spectra being 0.82 ± 0.32%16. The spectra (Fig. 3a) closely resemble those reported and visualized by Afara et al.42–44 with the most distinct spectral peak at 950 nm, resulting from second overtones of OH and NH stretching39,45.
All histological sections were analyzed via semi-automated software (MATLAB R2016b, MathWorks) in which all sections (DD, PLM, and FTIR) were manually inspected. This inspection ensured that: (1) all locations between modalities were matched, (2) no histological sections contained folded tissue, and (3) that no other mistakes were made during the histological processing. Mammals have a distinct structure of articular cartilage with no substantial differences between the species46,47; therefore, the comparison is justifiable. The presented profiles (Fig. 3d) of collagen content, proteoglycan content, and collagen orientation angle closely resemble those previously reported in literature31,48–51.
ISA-Tab metadata file
Acknowledgements
Doctoral Programme in Science, Technology and Computing (SCITECO) of University of Eastern Finland, Kuopio University Hospital (VTR projects 5041750, 5041744, 5041778, and 5203111, PY210 Clinical Neurophysiology), the Academy of Finland (projects 267551, 310466, and 315820), Orion Research Foundation sr, and Finnish foundation of technology promotion (project 8193) financially supported this study. Michael Amissah is acknowledged for support during data acquisition.
Online-only Table
Author Contributions
Jaakko K. Sarin, Principal author(*), data acquisition. Jari Torniainen, Principal author(*), data records and data validation. Mithilesh Prakash, data validation and data processing. Lassi Rieppo, data acquisition. Isaac Afara, data acquisition and supervision. Juha Töyräs, supervision. All authors contributed on study design and final manuscript.
Code Availability
The guidelines and example codes provided in the data description present good practices for preprocessing and analysing spectroscopic (NIR and FTIR) and biomechanical signal. The NIR spectroscopic data is validated through multivariate modelling (i.e., partial least squares regression) and cross-validation. In addition, the means of calculating the amide (collagen) and proteoglycan images from FTIR images are presented. The custom codes presented were written using MATLAB R2015–2017 (Mathworks Inc., Natick, MA, USA). Examples codes are available in a public repository (https://github.com/uef-bbc/sarin-scientific-data-2019).
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Jaakko K. Sarin and Jari Torniainen.
Contributor Information
Jaakko K. Sarin, Email: jaakko.sarin@uef.fi
Jari Torniainen, Email: jari.torniainen@uef.fi.
ISA-Tab metadata
is available for this paper at 10.1038/s41597-019-0170-y.
References
- 1.Buckwalter JA, Martin JA. Osteoarthritis. Adv. Drug Deliv. Rev. 2006;58:150–67. doi: 10.1016/j.addr.2006.01.006. [DOI] [PubMed] [Google Scholar]
- 2.Chu CR, Williams AA, Coyle CH, Bowers ME. Early diagnosis to enable early treatment of pre-osteoarthritis. Arthritis Res. Ther. 2012;14:212. doi: 10.1186/ar3845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gage BE, McIlvain NM, Collins CL, Fields SK, Comstock RD. Epidemiology of 6.6 million knee injuries presenting to United States emergency departments from 1999 through 2008. Acad. Emerg. Med. 2012;19:378–385. doi: 10.1111/j.1553-2712.2012.01315.x. [DOI] [PubMed] [Google Scholar]
- 4.Gelber AC, et al. Joint Injury in Young Adults and Risk for Subsequent Knee and Hip Osteoarthritis. Ann. Intern. Med. 2000;133:321–328. doi: 10.7326/0003-4819-133-5-200009050-00007. [DOI] [PubMed] [Google Scholar]
- 5.Brown TD, Johnston RC, Saltzman CL, Marsh JL, Buckwalter JA. Posttraumatic Osteoarthritis: A First Estimate of Incidence, Prevalence, and Burden of Disease. J. Orthop. Trauma. 2006;20:739–744. doi: 10.1097/01.bot.0000246468.80635.ef. [DOI] [PubMed] [Google Scholar]
- 6.Arden N, Nevitt MC. Osteoarthritis: Epidemiology. Best Practice and Research: Clinical Rheumatology. 2006;20:3–25. doi: 10.1016/j.berh.2005.09.007. [DOI] [PubMed] [Google Scholar]
- 7.Brittberg M, Winalski CS. Evaluation of cartilage injuries and repair. J. Bone Joint Surg. Am. 2003;85(A Suppl):58–69. doi: 10.2106/00004623-200300002-00008. [DOI] [PubMed] [Google Scholar]
- 8.Spahn G, Klinger HM, Hofmann GO. How valid is the arthroscopic diagnosis of cartilage lesions? Results of an opinion survey among highly experienced arthroscopic surgeons. Arch. Orthop. Trauma Surg. 2009;129:1117–1121. doi: 10.1007/s00402-009-0868-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Spahn G, Klinger HM, Baums M, Pinkepank U, Hofmann GO. Reliability in arthroscopic grading of cartilage lesions: results of a prospective blinded study for evaluation of inter-observer reliability. Arch. Orthop. Trauma Surg. 2011;131:377–381. doi: 10.1007/s00402-011-1259-8. [DOI] [PubMed] [Google Scholar]
- 10.Brismar BH, Wredmark T, Movin T, Leandersson J, Svensson O. Observer reliability in the arthroscopic classification of osteoarthritis of the knee. J. Bone Joint Surg. Br. 2002;84:42–47. doi: 10.1302/0301-620X.84B1.11660. [DOI] [PubMed] [Google Scholar]
- 11.Wang S-Z, Huang Y-P, Saarakkala S, Zheng Y-P. Quantitative assessment of articular cartilage with morphologic, acoustic and mechanical properties obtained using high-frequency ultrasound. Ultrasound Med. Biol. 2010;36:512–527. doi: 10.1016/j.ultrasmedbio.2009.12.005. [DOI] [PubMed] [Google Scholar]
- 12.Sarin JK, et al. Arthroscopic near infrared spectroscopy enables simultaneous quantitative evaluation of articular cartilage and subchondral bone in vivo. Sci. Rep. 2018;8:13409. doi: 10.1038/s41598-018-31670-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Saarakkala S, Wang S-Z, Huang Y-P, Zheng Y-P. Quantification of the optical surface reflection and surface roughness of articular cartilage using optical coherence tomography. Phys. Med. Biol. 2009;54:6837–6852. doi: 10.1088/0031-9155/54/22/006. [DOI] [PubMed] [Google Scholar]
- 14.McGoverin CM, et al. Nondestructive Assessment of Engineered Cartilage Composition by Near Infrared Spectroscopy. Ann. Biomed. Eng. 2016;44:680–692. doi: 10.1007/s10439-015-1536-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Afara IO, Prasadam I, Arabshahi Z, Xiao Y, Oloyede A. Monitoring osteoarthritis progression using near infrared (NIR) spectroscopy. Sci. Rep. 2017;7:11463. doi: 10.1038/s41598-017-11844-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sarin JK, et al. Near Infrared Spectroscopic Mapping of Functional Properties of Equine Articular Cartilage. Ann. Biomed. Eng. 2016;44:3335–3345. doi: 10.1007/s10439-016-1659-6. [DOI] [PubMed] [Google Scholar]
- 17.Sarin JK, et al. Combination of optical coherence tomography and near infrared spectroscopy enhances determination of articular cartilage composition and structure. Sci. Rep. 2017;7:10586. doi: 10.1038/s41598-017-10973-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Prakash M, Sarin JK, Rieppo L, Afara IO, Töyräs J. Optimal Regression Method for Near-Infrared Spectroscopic Evaluation of Articular Cartilage. Appl. Spectrosc. 2017;71:2253–2262. doi: 10.1177/0003702817726766. [DOI] [PubMed] [Google Scholar]
- 19.Prakash M, Sarin JK, Rieppo L, Afara IO, Töyräs J. Accounting for spatial dependency in multivariate spectroscopic data. Chemom. Intell. Lab. Syst. 2018;182:166–171. doi: 10.1016/j.chemolab.2018.09.010. [DOI] [Google Scholar]
- 20.NIR of Corn Samples for Standardization Bechmarking. Eigenvector Research, http://www.eigenvector.com/data/Corn/index.html (2005).
- 21.Pedersen, D. K., Martens, H., Nielsen, J. P. & Engelsen, S. B. Wheat Kernels. University of Copenhagen, http://www.models.life.ku.dk/wheat_kernels (2002).
- 22.Rinnan and Rinnan. Soil samples measured by NIR, with two reference values. University of Copenhagen, http://www.models.life.ku.dk/NIRsoil (2007).
- 23.Conference International Diffure Reflectance. NIR Spectra of Pharmaceutical Tablets from ‘Shootout’. Eigenvector Research, http://www.eigenvector.com/data/tablets/index.html (2002).
- 24.Dyrby, M., Engelsen, S. B., Nørgaard, L., Burhn, M. & Lundsberg Nielsen, L. Active substance in pharmaceutical tablets. University of Copenhagen, http://www.models.life.ku.dk/Tablets (2002).
- 25.Southwest Research Institute (SWRI). Near Infrared Spectra of Diesel Fuels. Eigenvector Research, http://www.eigenvector.com/data/SWRI/index.html#csv (2005).
- 26.Christensen, J., Nørgaard, L., Heimdal, H., Pedersen, J. G. & Engelsen, S. B. Data sets for multi-variate data analysis. University of Copenhagen, http://www.models.life.ku.dk/Marzipan (2004).
- 27.Thybo, A. K., Bechmann, I. E., Martens, M. & Engelsen, S. B. Sensory and physical (uniaxial compression, NIR, LF-NMR) texture measurement of potatoes. University of Copenhagen, http://www.models.life.ku.dk/Texture_Potatoes (2000).
- 28.O’Hare LMS, Cox PG, Jeffery N, Singer ER. Finite element analysis of stress in the equine proximal phalanx. Equine Vet. J. 2013;45:273–277. doi: 10.1111/j.2042-3306.2012.00635.x. [DOI] [PubMed] [Google Scholar]
- 29.McGoverin CM, Lewis K, Yang X, Bostrom MPG, Pleshko N. The Contribution of Bone and Cartilage to the Near-Infrared Spectrum of Osteochondral Tissue. Appl. Spectrosc. 2014;68:1168–1175. doi: 10.1366/13-07327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rinnan Å, Berg Fvanden, Engelsen SB. Review of the most common pre-processing techniques for near-infrared spectra. TrAC Trends Anal. Chem. 2009;28:1201–1222. doi: 10.1016/j.trac.2009.07.007. [DOI] [Google Scholar]
- 31.Rieppo L, et al. Application of second derivative spectroscopy for increasing molecular specificity of fourier transform infrared spectroscopic imaging of articular cartilage. Osteoarthr. Cartil. 2012;20:451–459. doi: 10.1016/j.joca.2012.01.010. [DOI] [PubMed] [Google Scholar]
- 32.Hayes WC, Keer LM, Herrmann G, Mockros LF. A mathematical analysis for indentation tests of articular cartilage. J. Biomech. 1972;5:541–551. doi: 10.1016/0021-9290(72)90010-3. [DOI] [PubMed] [Google Scholar]
- 33.Korhonen R, et al. Comparison of the equilibrium response of articular cartilage in unconfined compression, confined compression and indentation. J. Biomech. 2002;35:903–909. doi: 10.1016/S0021-9290(02)00052-0. [DOI] [PubMed] [Google Scholar]
- 34.Király K, et al. Safranin O reduces loss of glycosaminoglycans from bovine articular cartilage during histological specimen preparation. Histochem. J. 1996;28:99–107. doi: 10.1007/BF02331414. [DOI] [PubMed] [Google Scholar]
- 35.Király K, et al. Application of selected cationic dyes for the semiquantitative estimation of glycosaminoglycans in histological sections of articular cartilage by microspectrophotometry. Histochem. J. 1996;28:577–590. doi: 10.1007/BF02331378. [DOI] [PubMed] [Google Scholar]
- 36.Király K, et al. Specimen preparation and quantification of collagen birefringence in unstained sections of articular cartilage using image analysis and polarizing light microscopy. Histochem. J. 1997;29:317–327. doi: 10.1023/A:1020802631968. [DOI] [PubMed] [Google Scholar]
- 37.Rieppo L, et al. Infrared spectroscopic analysis of human and bovine articular cartilage proteoglycans using carbohydrate peak or its second derivative. J. Biomed. Opt. 2013;18:097006. doi: 10.1117/1.JBO.18.9.097006. [DOI] [PubMed] [Google Scholar]
- 38.Sarin JK, 2019. Dataset on equine cartilage near infrared spectra, composition, and functional properties. figshare. [DOI] [PMC free article] [PubMed]
- 39.Burns DA, Ciurczak EW. Handbook of near-infrared analysis, 3rd ed. Analytical and Bioanalytical Chemistry. 2009;393:1387–1389. doi: 10.1007/s00216-009-2644-9. [DOI] [Google Scholar]
- 40.Brommer H, et al. Functional consequences of cartilage degeneration in the equine metacarpophalangeal joint: quantitative assessment of cartilage stiffness. Equine Vet. J. 2005;37:462–7. doi: 10.2746/042516405774480012. [DOI] [PubMed] [Google Scholar]
- 41.Cook JL, et al. Animal models of cartilage repair. Bone Joint Res. 2014;3:89–94. doi: 10.1302/2046-3758.34.2000238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Afara IO, Singh S, Oloyede A. Load-unloading response of intact and artificially degraded articular cartilage correlated with near infrared (NIR) absorption spectra. J. Mech. Behav. Biomed. Mater. 2013;20:249–58. doi: 10.1016/j.jmbbm.2012.11.022. [DOI] [PubMed] [Google Scholar]
- 43.Afara IO, et al. Near infrared spectroscopy for rapid determination of Mankin score components: a potential tool for quantitative characterization of articular cartilage at surgery. Arthroscopy. 2014;30:1146–55. doi: 10.1016/j.arthro.2014.04.097. [DOI] [PubMed] [Google Scholar]
- 44.Afara IO, Prasadam I, Crawford R, Xiao Y, Oloyede A. Non-destructive evaluation of articular cartilage defects using near-infrared (NIR) spectroscopy in osteoarthritic rat models and its direct relation to Mankin score. Osteoarthritis Cartilage. 2012;20:1367–73. doi: 10.1016/j.joca.2012.07.007. [DOI] [PubMed] [Google Scholar]
- 45.Stuart, Barbara. H. Infrared Spectroscopy: Fundamentals and applications. John Wiley & Sons Ltd, ISBN: 978-470-85428-0, 244 (2004).
- 46.Malda J, et al. Of Mice, Men and Elephants: The Relation between Articular Cartilage Thickness and Body Mass. PLoS One. 2013;8:e57683. doi: 10.1371/journal.pone.0057683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Malda J, et al. Comparative study of depth-dependent characteristics of equine and human osteochondral tissue from the medial and lateral femoral condyles. Osteoarthr. Cartil. 2012;20:1147–1151. doi: 10.1016/j.joca.2012.06.005. [DOI] [PubMed] [Google Scholar]
- 48.Mäkelä JTA, et al. Site-dependent changes in structure and function of lapine articular cartilage 4 weeks after anterior cruciate ligament transection. Osteoarthritis Cartilage. 2014;22:869–878. doi: 10.1016/j.joca.2014.04.010. [DOI] [PubMed] [Google Scholar]
- 49.Saarakkala S, et al. Depth-wise progression of osteoarthritis in human articular cartilage: investigation of composition, structure and biomechanics. Osteoarthritis Cartilage. 2010;18:73–81. doi: 10.1016/j.joca.2009.08.003. [DOI] [PubMed] [Google Scholar]
- 50.Oinas J, et al. Imaging of Osteoarthritic Human Articular Cartilage using Fourier Transform Infrared Microspectroscopy Combined with Multivariate and Univariate Analysis. Sci. Rep. 2016;6:30008. doi: 10.1038/srep30008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Oinas J, et al. Composition, structure and tensile biomechanical properties of equine articular cartilage during growth and maturation. Sci. Rep. 2018;8:11357. doi: 10.1038/s41598-018-29655-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Sarin JK, 2019. Dataset on equine cartilage near infrared spectra, composition, and functional properties. figshare. [DOI] [PMC free article] [PubMed]
Supplementary Materials
Data Availability Statement
The guidelines and example codes provided in the data description present good practices for preprocessing and analysing spectroscopic (NIR and FTIR) and biomechanical signal. The NIR spectroscopic data is validated through multivariate modelling (i.e., partial least squares regression) and cross-validation. In addition, the means of calculating the amide (collagen) and proteoglycan images from FTIR images are presented. The custom codes presented were written using MATLAB R2015–2017 (Mathworks Inc., Natick, MA, USA). Examples codes are available in a public repository (https://github.com/uef-bbc/sarin-scientific-data-2019).