Fig. 1.
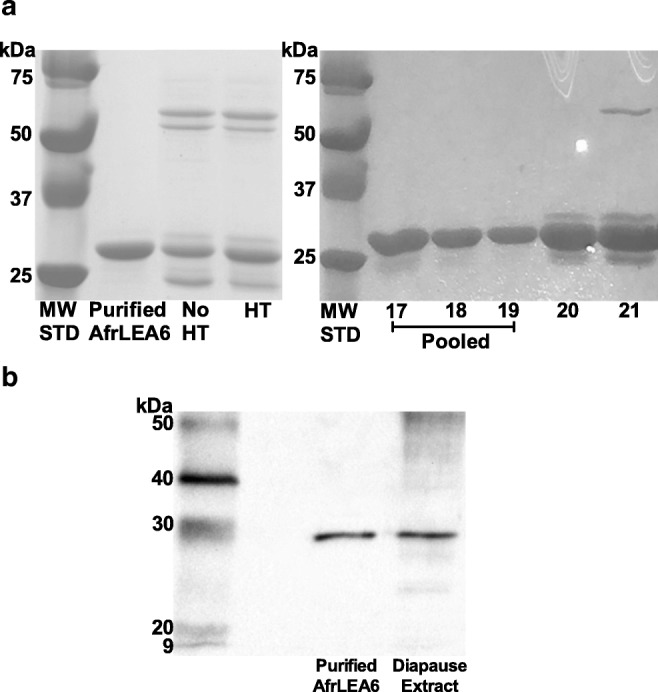
Purification of recombinant AfrLEA6. aLeft panel: SDS-PAGE analysis of purified protein after IMPACT column chromatography combined with anion exchange chromatography. Evidence that heat treatment (HT) is ineffective for removing contaminants present after IMPACT chromatography alone. Total protein loaded per lane was 40 μg. MW STD, ladder of molecular weight standards. Right panel: SDS-PAGE analysis of 1-ml fractions eluted from the anion exchange column (HiTrap Q FF resin) that contained AfrLEA6. Contamination is present in the trailing edge of the peak, which was not pooled. Each lane was loaded with 10 μl of eluant. b Western blot of purified, recombinant AfrLEA6 versus the native protein in a diapause embryo extract shows equivalent migration. Approximately 40 ng of purified AfrLEA6 and 40 μg total protein from an embryo extract prepared in Laemmli buffer were loaded in respective lanes. Ladder of MW standards shown at left
