Significance
By performing a targeted genetic screen of temperature-sensitive mutations, this study identified 94 essential Saccharomyces cerevisiae genome instability suppressing (eGIS) genes and 38 candidate eGIS genes. Analysis of The Cancer Genome Atlas data demonstrated that mutations in the human homologues of the S. cerevisiae eGIS genes were significantly enriched in 10 different human cancers. These results provide insights into the origin of genome instability in human cancers and provide tools for identifying and evaluating mutations that contribute to the development of cancer.
Keywords: genome instability, chromosome dynamics and replication, cancer
Abstract
Gross Chromosomal Rearrangements (GCRs) play an important role in human diseases, including cancer. Although most of the nonessential Genome Instability Suppressing (GIS) genes in Saccharomyces cerevisiae are known, the essential genes in which mutations can cause increased GCR rates are not well understood. Here 2 S. cerevisiae GCR assays were used to screen a targeted collection of temperature-sensitive mutants to identify mutations that caused increased GCR rates. This identified 94 essential GIS (eGIS) genes in which mutations cause increased GCR rates and 38 candidate eGIS genes that encode eGIS1 protein-interacting or family member proteins. Analysis of TCGA data using the human genes predicted to encode the proteins and protein complexes implicated by the S. cerevisiae eGIS genes revealed a significant enrichment of mutations affecting predicted human eGIS genes in 10 of the 16 cancers analyzed.
The genetic instability that occurs in many cancers is thought to play a critical role in the development and progression of tumors and falls into 3 general categories (1, 2): accumulation of mutations resulting from environmental mutagens, defects in DNA mismatch repair genes, defects that reduce the fidelity of DNA polymerases, and increased levels of cytosine deaminases (3–6); accumulation of genome rearrangements such as translocations and copy number changes (2, 7); and accumulation of changes in chromosome number (8). Our understanding of the genes that suppress genome rearrangements in cancer comes from the study of inherited defects causing cancer susceptibility syndromes such as Fanconi anemia and the BRCA1- and BRCA2-defective breast and ovarian cancer syndromes (9, 10). In addition, cancer genome sequencing projects have identified mutations in candidate Genome Instability Suppressing (GIS) genes, most of which were identified in studies of model organisms (11). However, our understanding of the causes of genome rearrangements in mammalian cells is incomplete in part because it is difficult to perform genetic screens to identify and study GIS genes in mammalian cells.
Genetic studies in Saccharomyces cerevisiae have provided considerable insight into mechanisms that promote and prevent spontaneous genome rearrangements (12). Such studies were made possible by the development of quantitative genetic assays that allow measurement of the rate of accumulation of Gross Chromosomal Rearrangements (GCRs) (13–18) and allow detection of a diversity of types of GCRs (13, 14, 19–24). Overall, the types of genome rearrangements selected in GCR assays resemble those seen in human diseases, including cancer (discussed in ref. 12). In addition, GCR assays have been used to identify genes that prevent GCRs and that alter the types of GCRs formed (13–17, 22, 25–34). These studies have shown that a combination of oxidative defense, DNA replication machinery, DNA repair, cell cycle checkpoint, telomere maintenance, RNA processing, and chromatin modification/remodeling and assembly function in concert to prevent GCRs (12).
Most genes and pathways that prevent and form GCRs in model organisms have been identified through analysis of nonessential genes (12–15, 17, 22, 25, 26, 35, 36). These studies have identified 182 genes that suppress increased GCR rates and 438 cooperating GIS genes, in which mutations do not cause increased GCR rates but only cause increased GCR rates when combined with mutations in other genes. In contrast, studies of essential genes have thus far identified only 29 essential genes in which defects cause increased GCR rates (13, 14, 17, 25, 26, 29, 35, 37–45). Here, we used 2 different GCR assays to screen a collection of temperature-sensitive (ts) mutants for mutations that cause increased GCR rates and identified 94 essential S. cerevisiae GIS (called eGIS) genes, of which 71 were not previously reported, as well as an additional 38 candidate eGIS genes, and analysis of The Cancer Genome Atlas (TCGA) data (46) demonstrated a significant enrichment of mutations affecting 1 or more predicted human eGIS genes in 10 of 16 cancers analyzed.
Results
A Genetic Screen to Identify Essential GIS Genes.
To identify essential genes that suppress the formation of GCRs, we crossed query strains containing either the duplication-mediated GCR (dGCR) assay or the short repeat-sequence-mediated (sGCR) assay (SI Appendix, Fig. S1A) with 412 ts mutants [tsV6 (47); provided by Charlie Boone] and a leu2Δ::kanMX4 control strain (11). The 412 ts mutations analyzed affected 248 genes involved in DNA replication, DNA damage response and repair, telomere maintenance, chromatin modification and remodeling, and chromosome cohesion, condensation, and segregation, as well as related pathways implicated in maintaining genome stability, including sumoylation, cell cycle, mitosis and cytokinesis, transcription, and nuclear envelope and nucleo-cytoplasmic transport (Dataset S1). We recovered GCR-assay containing progeny for 399 of the 412 ts mutant alleles (243 of the 248 genes) in crosses with at least 1 and usually both query strains.
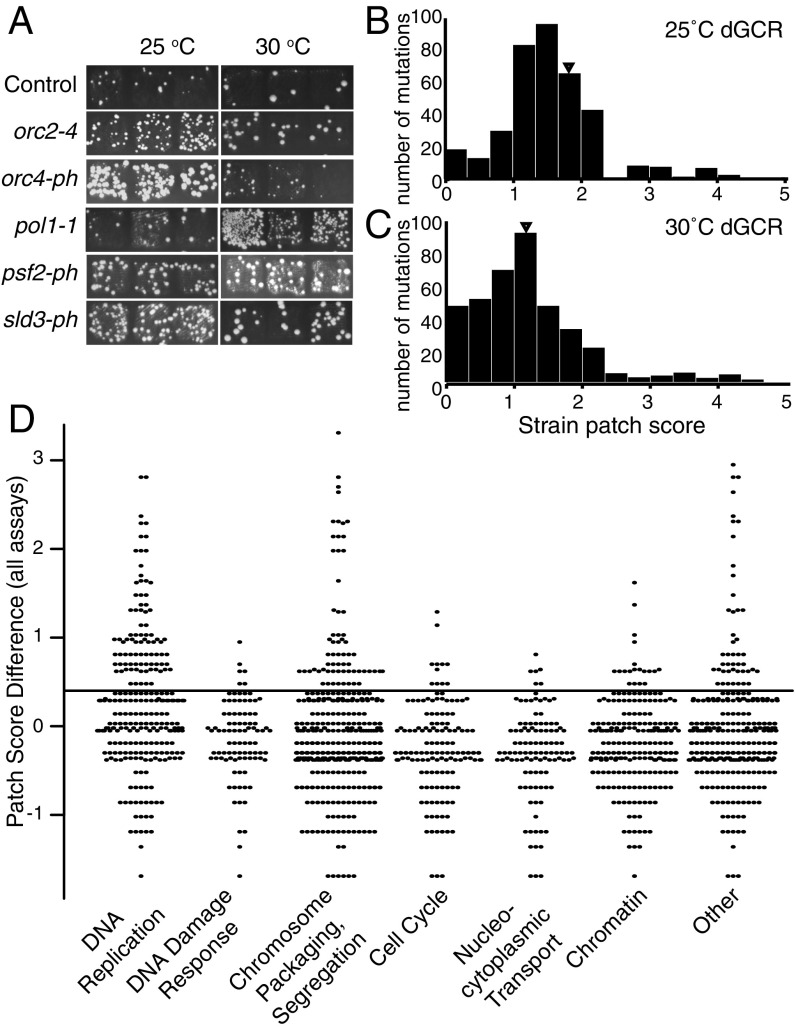
The progeny were scored using a papillation assay (11) at 30 °C and 25 °C (Fig. 1A). The number of papillae growing on medium that selected for GCRs was converted to a patch score ranging from 0 to 5 (11). At 25 °C and 30 °C, the leu2Δ control strain had average patch scores of 1.69 and 1.19, respectively, in the dGCR assay, and 0.38 and 0.30, respectively, in the sGCR assay (Fig. 1 B and C and SI Appendix, Fig. S1 B and C). To minimize false-positive and false-negative identification of GIS genes (11), we used a cutoff score difference of 0.4 above the leu2Δ control score at each temperature and identified 134 alleles of 103 genes with increased patch scores in at least 1 assay at least at 1 temperature. Some mutations appeared to cause assay-specific increased GCR patch scores; however, analysis that is beyond the scope of the current study will be required to verify this. The ts alleles affecting genes in the categories of DNA replication, chromosome cohesion, condensation, segregation, and other had the largest effect on patch scores (Fig. 1D and Dataset S1).
Fig. 1.
Identification of essential genome instability suppressing (eGIS) genes in S. cerevisiae. (A) Example patches of haploid strains containing the dGCR assay at permissive (25 °C) and semipermissive (30 °C) temperatures after replica plating onto GCR-selecting media. (B and C) Histograms of average strain patch scores for the dGCR assay at 25 °C (B) and 30 °C (C). The triangle indicates the position of the average patch score of the control (leu2Δ) strain. (D) Beehive plot of the difference in average patch score for each mutant strain relative to the control strain for the dGCR and sGCR assays measured at 25 °C and 30 °C (Dataset S1); an increase of 0.4 (horizontal line) was previously established as the cutoff for significance (11). Mutant strains were classified by the function of the affected gene.
Growth defects can potentially result in decreased GCR scores in papillation assays. We therefore validated mutations affecting each pathway implicated by the papillation assays by measuring quantitative GCR rates (Dataset S1). This excluded 18 alleles that caused elevated patch scores but not increased GCR rates. This also identified 12 alleles that caused increased GCR rates but not elevated patch scores. Finally, we sequenced all alleles of interest and eliminated 10 strains lacking the expected mutations (SI Appendix, Table S1). In total, we identified 121 ts alleles representing 94 eGIS genes (Dataset S1).
Definition of the eGIS1 and eGIS2 Gene Lists.
The 94 essential genes identified defined the first version of the eGIS gene list (eGIS1; Dataset S1), which included genes related to DNA replication and the sister chromatid cohesion, chromosome condensation, and chromosome segregation pathways. Seventy-one of the eGIS1 genes had no previously known role in suppressing GCRs. Examples include IPI1, which has a role in ribosome biogenesis and is potentially a component of the prereplication complex (48, 49), and SLD3, which is required for the activation of the MCM replicative DNA helicase (50). Other newly identified eGIS1 genes encoded cell cycle-related proteins (such as Apc4, component of the anaphase-promoting complex); Cdc15 (required for mitotic exit); Cdc4 (F-box protein required for cell cycle transitions); Cdc34 (ubiquitin-conjugating enzyme); Cdc37 (chaperone needed for passage through START during the cell cycle); 20S and 26S proteasome-related proteins Pre2, Pre6, Pre10, Rpn5, and Rpt6; and kinetochore and spindle-related proteins Mif2, Nnf1, Nuf2, and Spc42.
Seven previously implicated genes (DNA2, POL30, SPN1, SUA7, TAF4, TOA1, and TFG1) were not present in the eGIS1 list (26, 39, 41, 45): 5 genes (POL30, SUA7, TAF4, TOA1, and TFG1) were not represented in the mutation collection screened here, and 2 genes (DNA2, SPN1) were identified as GIS genes using alleles that were not tested here (dna2-2, TET-SPN1). Because of the lack of alleles in our mutation collection affecting all subunits or pathway components implicated by the eGIS1 genes and the potential for allele-specific effects on GCR rates, we created the expanded eGIS2 list (Dataset S1), which additionally included 38 genes encoding other components of the complexes and pathways defined by the eGIS1 genes. To be stringent, we only added genes encoding components of complexes in which a high proportion of the genes encoding the complex were identified as eGIS1 genes; for example, we identified 4 MCM genes as eGIS1 genes and added the 2 remaining MCM genes.
Identification of Mutations That Activate the DNA Damage Response.
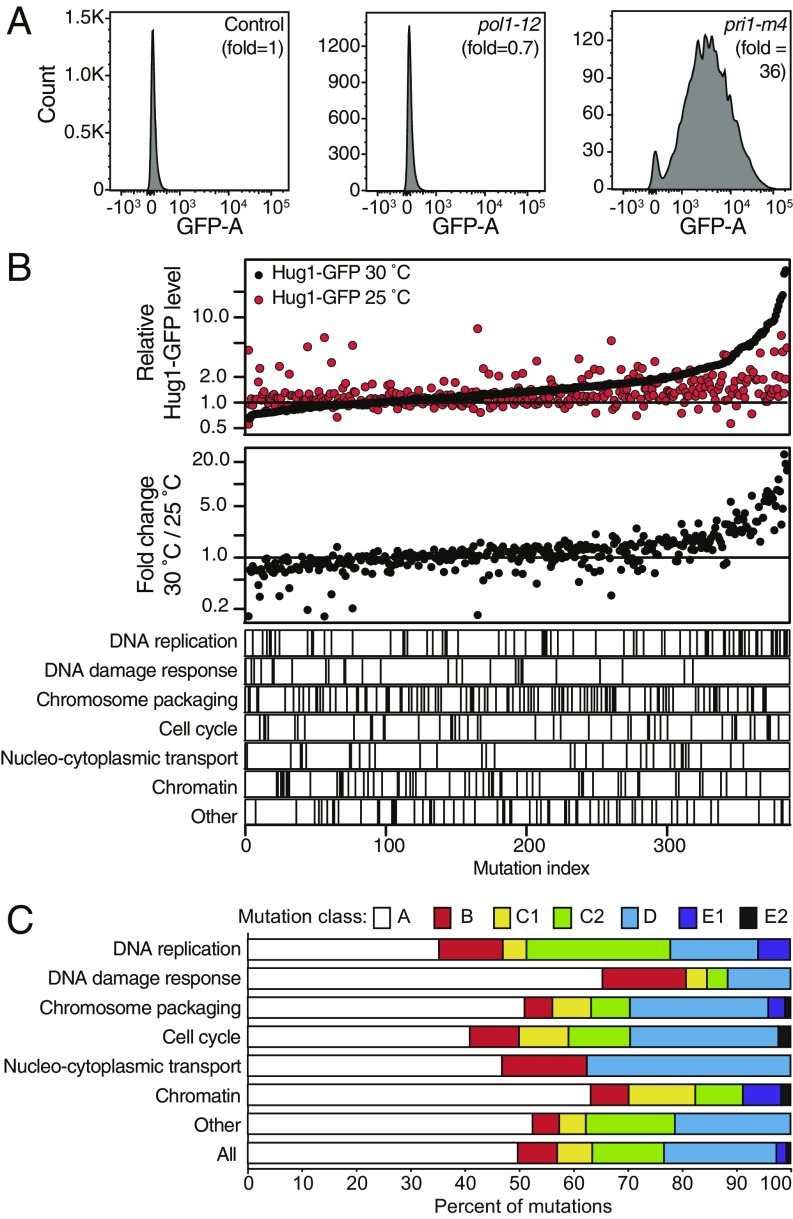
The formation of GCRs involves aberrant processing of damaged chromosomes (12). To distinguish between eGIS gene mutations that directly or indirectly disrupt normal DNA damage processing from those that increase the levels of damaged chromosomes, we introduced the HUG1-GFP reporter, whose expression is induced by activation of the DNA damage and replication checkpoints (51), into the starting 413 mutant strains. We measured the fold-increase in Hug1-GFP levels at 25 °C and 30 °C, using FACS (Fig. 2A and ref. 52). Multiple mutations caused dramatically increased Hug1-GFP levels (Fig. 2B), including those affecting DNA replication complexes (DNA polymerase alpha/primase, origin recognition, replication factor A, the GINS complex, and Okazaki fragment maturation), cell-cycle complexes (the RENT complex, the anaphase promoting complex/cyclosome, and cyclin-dependent protein kinase), and chromosome packaging complexes (cohesin, the cohesin loader, and the Smc5-Smc6 complex). In addition, defects in sumoylation (“other” category) were also identified.
Fig. 2.
Monitoring induction of the DNA damage response by FACS using the Hug1-GFP reporter. (A) Example histograms of Hug1-GFP levels (GFP-Area signals) as a function of the number of FACS events for the control (leu2Δ), pol1-12, and pri1-m4 strains at 30 °C. Fold changes are the mutant mean GFP-Area signal divided by the control mean GFP-Area signal. (B, Top) Summary of the changes of Hug1-GFP levels for the mutant strains at 25 °C (red) and 30 °C (black) rank ordered by the change at 30 °C. (B, Middle) Ratio of the fold changes at 30 °C relative to 25 °C shows that ts allele-containing strains with the highest Hug1-GFP levels at 30 °C tend to be induced by increased temperature. (B, Bottom) Position of ts alleles affecting different processes in the rank ordered list indicated by vertical lines; DNA replication, chromosome packaging, and segregation, cell cycle, and other categories dominate the alleles with the highest Hug1-GFP levels at 30 °C. (C) Distribution of the ts alleles for different processes based on the Hug1-GFP levels (class A = no increase, class B = increase at 25 °C, class C1 and C2 = increase at 25 °C and 30 °C, class D = increase at 30 °C, class E = missing data; see Identification of Mutations That Activate the DNA Damage Response).
We divided the mutant strains into 5 categories (Fig. 2C and Dataset S1): those that did not cause increased Hug1-GFP levels (class A, 192 mutations), those that caused increased Hug1-GFP levels only at 25 °C (class B, 28 mutations), at both 25 °C and 30 °C (class C, 76 mutations), only at 30 °C (class D, 80 mutations), or those for which data were only available at 1 temperature due to growth defects (class E, 10 mutations). Class C mutations included those whose Hug1-GFP levels were relatively temperature-insensitive (class C1, 25 mutations) and those whose Hug1-GFP levels were higher at 30 °C relative to 25 °C (class C2, 51 mutations). Class E mutations included those with data available only at 25 °C (class E1, 7 mutations) or at 30 °C (class E2, 3 mutations). Different ts mutations affecting the same protein or complex belong to different classes; for example, the 6 POL1 alleles belonged to classes A, C1, C2, and D. Increased Hug1-GFP levels were observed in at least 1 temperature for 50.3% of the alleles (142 of 244 genes), and 33.9% of the alleles (106 of 244 genes) had increased Hug1-GFP levels at 30 °C compared with 25 °C. More than 50% of the tested mutations affecting DNA replication, chromosome packaging and segregation, cell cycle, and nucleo-cytoplasmic transport categories caused increased Hug1-GFP levels (Fig. 2C); the relatively low proportion of ts mutations affecting the DNA damage category that caused increased Hug1-GFP levels is likely because many of the affected genes play modest roles in promoting DNA repair.
We tested the effect of temperature on the accumulation of GCRs for a subset of the 27 C2 and D mutations with at least a 3-fold increase in Hug1-GFP levels at 30 °C compared with that at 25 °C (Dataset S1 and SI Appendix, Table S2). The pol1-1 and cdc9-1 mutants had a dramatic increase in GCR rate when shifted from 25 °C to 30 °C. In contrast, the mcm10-1 GCR rate was not temperature dependent, and smc1-259 and nuf2-61 mutants had reduced GCR rates at the higher temperature. Thus, temperature-dependent changes in the DNA damage response as measured by Hug1-GFP levels did not always correlate with temperature-dependent increases in GCR rates. This may be because the formation of GCRs requires both the generation and misrepair of DNA damage, and in some mutants the increased DNA damage may not be repairable, resulting in no change or even reduced changes in GCR rates or cell death. Regardless, there was a strong correlation in general between defects causing increased levels of Hug1-GFP and those causing increased accumulation of GCRs (Fig. 3).
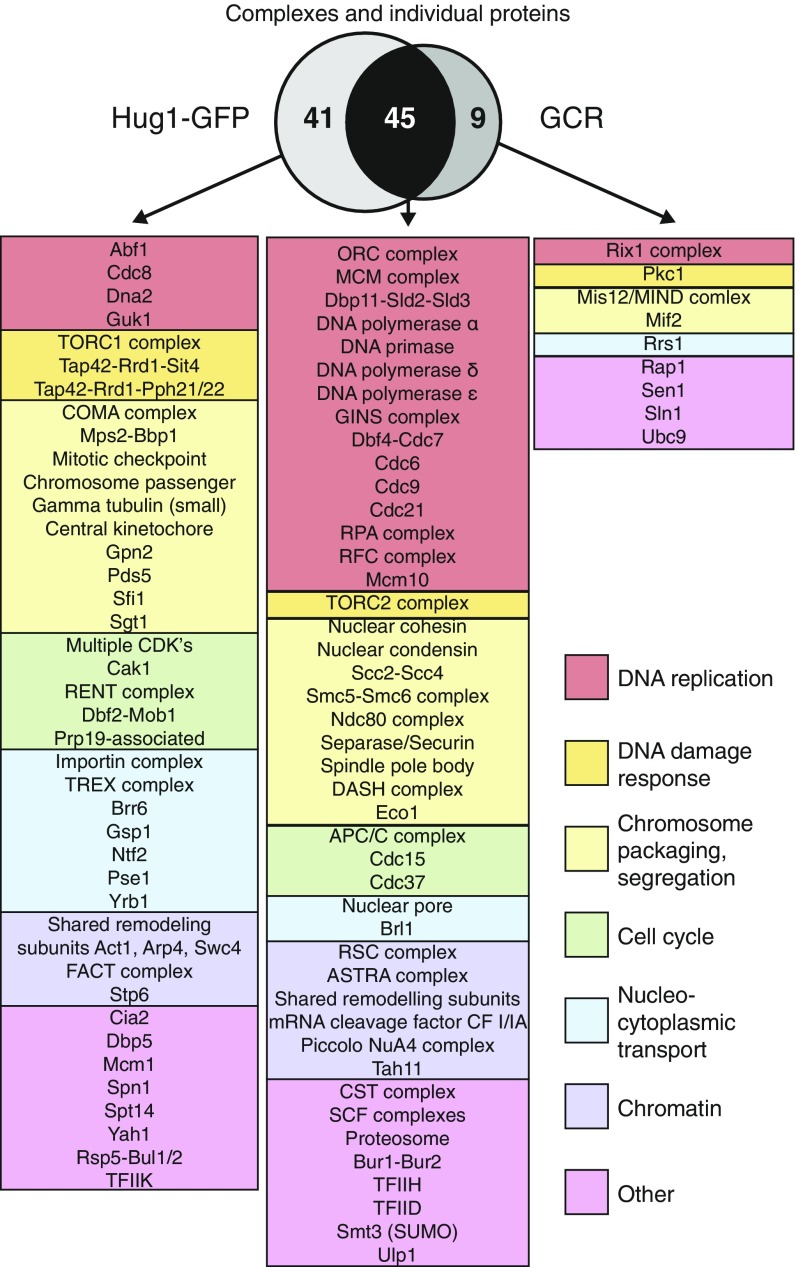
Fig. 3.
Comparison of mutations causing increased Hug1-GFP signaling and increased GCR rates. Alleles causing increased Hug1-GFP were mapped to protein complexes or individual proteins on the basis of the affected genes. The resulting 95 complexes/proteins were divided into those only affecting Hug1-GFP signaling, those only affecting GCR rates, or those affecting both. The complexes/proteins were then color-coded by biological process.
Mutation of eGIS Genes in Human Cancers.
To examine whether eGIS genes were inactivated in human cancers, we first generated the human eGIS1 (heGIS1) gene list (115 genes; Datasets S1 and S2), which corresponded to human homologs of the S. cerevisiae eGIS1 genes, and the heGIS2 gene list (162 genes; Datasets S1 and S3), which contained the human homologs of the S. cerevisiae eGIS2 genes and 2 additional human genes (CDCA5 and POT1) that lacked S. cerevisiae homologs but encoded proteins that function in the pathways identified by the S. cerevisiae analysis. We then identified the mutations in these genes in the TCGA data for 16 different cancers (Datasets S2–S5) and computationally analyzed the heGIS1 and heGIS2 gene lists for significant enrichment of mutations (Fig. 4A; see Methods). The mutations included in the analysis were loss-of-function (LOF) mutations (nonsense mutations, frameshift insertions/deletions, and splice-site mutations) or LOF plus missense mutations (Datasets S2–S5 and SI Appendix, Table S3); data from analysis of specific classes of mutations are present in Datasets S2 and S3 and SI Appendix, Tables S4–S12. We also calculated S-scores for each heGIS1 and heGIS2 gene and performed enrichment analysis (11).
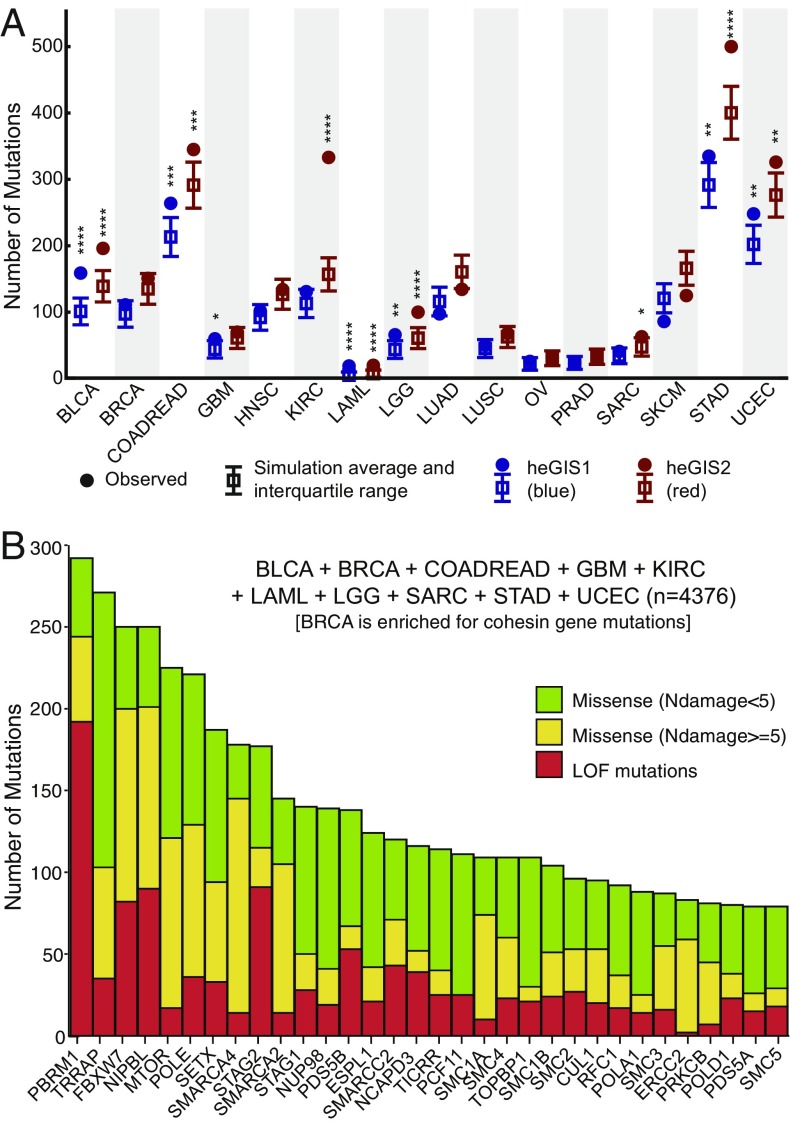
Fig. 4.
Analysis of TCGA data for mutations in human homologs of S. cerevisiae eGIS genes. (A) Summary of the simulations to determine whether human homologs of the eGIS1 and eGIS2 genes are significantly mutated in cancers sequenced by the TCGA. Solid circles are the observed number of loss-of-function and missense mutations for the heGIS1 and heGIS2 gene lists. The box and whiskers correspond the average and interquartile range from the in silico simulations. Statistically significant P values are indicated by the number of asterisks (4 = P < 0.0001, 3 = P < 0.001, 2 = P < 0.01, 1 = P < 0.05). All significant P values were above a false-discovery rate of 0.05 as determined by the Benjamini-Hochberg procedure. (B) Count of the number of mutations in the top 50 mutated heGIS2 genes from the 9 cancers with significant levels of mutations and BRCA, which is enriched for cohesin gene mutations.
Significant enrichment of mutations in a broad array of human eGIS genes were observed in 9 of the 16 TCGA cancers analyzed: bladder urothelial carcinoma, colorectal adenocarcinoma, glioblastoma multiforme (GBM), kidney renal clear cell carcinoma, acute myeloid leukemia (LAML), low-grade glioma, sarcoma, stomach adenocarcinoma, and uterine corpus endometrial carcinoma (UCEC; Fig. 4 and SI Appendix, Tables S3–S12). Enrichment in GBM was specific to heGIS1, and enrichment in kidney renal clear cell carcinoma and sarcoma was specific to heGIS2. Among these 9 cancer types, bladder urothelial carcinoma samples had the greatest incidence of mutations in heGIS genes (SI Appendix, Fig. S2), and LAML, a cancer that has limited genome instability, had the lowest incidence of mutations in the expanded heGIS2 gene set and among the lowest incidence in the heGIS1 gene set. This is consistent with our previous study, in which LAML was not significantly enriched in mutations in nonessential GIS genes (11). Breast invasive carcinoma (BRCA) only showed significant enrichment for mutations in cohesion genes. In contrast to these 10 cancers, no enrichment for mutations was observed with either gene list in head-neck squamous cell carcinoma, lung adenocarcinoma, lung squamous cell carcinoma, ovarian serous cystadenocarcinoma, prostate adenocarcinoma, and skin cutaneous melanoma. We also found that there was significant enrichment for genes with S-score >2 and >3 among the heGIS1 genes in 11 cancers and among heGIS2 genes in 12 cancers (SI Appendix, Table S13); as expression changes are an important component in the S-score calculation (11), this suggests there may be overexpression of individual GIS genes in different cancers, although we did not analyze this.
Overall, genes encoding replication-related complexes, the cohesin and condensin complexes, and chromatin- and transcription-related complexes were among the most frequently mutated genes in the 10 cancers that showed enrichment for mutations in the complete heGIS gene lists or a subset of heGIS genes (Fig. 4B). For example, 11.7% of bladder urothelial carcinoma samples contained a mutation or mutations in STAG2, and NIPBL mutation or mutations were observed in 19.8% of UCEC samples, 8.5% of stomach adenocarcinoma samples, 4.5% of low-grade glioma samples, and 1.6% of BRCA samples (Datasets S2–S5). In BRCA, GBM, and LAML, the overall enrichment was entirely attributable to mutations in cohesin genes. Other frequently mutated genes included MTOR (mammalian target of rapamycin), SETX (senataxin), FBXW (F-box/WD-repeat containing protein 7), and POLE (catalytic subunit of DNA polymerase epsilon). Both FBXW and POLE contain recurrent missense mutations in addition to other loss-of-function mutations; the recurrent POLE mutations cause defects in the proofreading exonuclease activity (53). Given the importance of the SMC5/6 complex in DNA repair, it was surprising that mutations in genes encoding the SMC5/6 complex were only significantly enriched in 1 cancer type (UCEC) (SI Appendix, Table S3).
Discussion
Here, we performed a targeted screen for mutations in essential genes that result in increased GCR rates. We focused on mutations affecting genes involved in DNA and chromosome metabolism, cell cycle regulation, and other processes previously implicated in maintaining genome stability (12). These mutations were screened for those causing increased GCR rates at 2 different temperatures using 2 different assays that detect different but overlapping types of GCRs. Use of the 2 assays and 2 different temperatures increased the number of tests performed, and hence the sensitivity of the screen, and allowed identification of mutations that preferentially affect specific types of GCRs (15). This screen identified 121 mutations in 94 essential GIS genes, 71 of which have not been previously reported. The 94 eGIS genes encode 47 multiprotein complexes (78 genes) and 16 proteins that function as individual proteins (16 genes); this list of genes is referred to as eGIS1. In many cases, 1 or more genes encoding a protein complex were identified as GIS genes, but not all of the genes encoding the complex were identified as GIS genes. The most likely explanation for this is that our mutation collection did not contain a sufficient number of mutations in each gene to allow identification of all possible mutations that could cause a defect resulting in increased GCR rates. We therefore constructed the eGIS2 gene list comprising 132 genes, which also contained other genes encoding protein complexes implicated in suppressing GCRs that were not present in the eGIS1 list (see Definition of the eGIS1 and eGIS2 Gene Lists). In combination with the previously identified nonessential GIS genes (11), the genes in eGIS1 and eGIS2 identify 266 and 304 GIS1 and GIS2 genes, respectively.
The eGIS genes identified have implicated a number of metabolic processes in the suppression of GCRs. The most prominent group of genes identified was those encoding DNA replication factors; these included most replication proteins including ORC, the MCM helicase, GINS proteins, all the DNA polymerases, DNA primase, RFA, RFC, and various proteins involved in control of DNA replication. This is consistent with previous studies implicating replication errors in the production of GCRs and studies showing that down-regulation of DNA polymerases can result in increased formation of GCRs (13, 54). A second prominent group of GIS genes were those encoding cohesin, condensin, and the Smc5-Smc6 cohesion complex; the latter has been extensively studied in regard to its role in DNA repair (29) and plays a role in suppressing GCRs (29, 40). Among other functions, cohesin acts during DNA replication to maintain sister chromatid cohesion, which plays a role in sister chromatid recombination (55), a process suggested to suppress GCRs (12, 26). Condensin plays a role in maintaining the 3D structure of chromosomes (55), but how this functions to suppress GCRs is not clear; regardless, condensin defects caused similar increases in GCR rates as those caused by defects in cohesin and the Smc5-Smc6 cohesin complex (this study and refs. 29 and 40). We also found a modest number of mutations affecting the proteasome (56), sumoylation pathways (35, 57), chromatin remodeling complexes (52, 58), and nuclear pore (59), all of which act in DNA repair and the DNA damage response to some extent. Among the more surprising GIS genes identified were those encoding the TORC2 complex that may play a role in DNA damage responses (60); Senataxin, which acts in R-loop regulation (61); and Pkc1, which plays a minor role in DNA damage checkpoint responses (62).
To better classify the eGIS genes, we included a Hug1-GFP downstream transcriptional reporter of the DNA damage response in our screen (51, 52). This allowed the identification of 3 classes of genes. The first class was those in which defects caused increased Hug1 expression but no increase in GCR rates; defects in these genes are likely to directly affect the transcription of DNA damage response genes and are unlikely to reflect processes that suppress GCRs or possibly cause increased levels of DNA damage that is repaired in ways that do not result in the formation of GCRs. The second, and most prevalent, class was those in which defects caused both increased Hug1 expression and increased GCR rates and encompassed virtually all of the replication and cohesin/condensin genes. It seems likely that defects in replication genes result in increased levels of DNA damage because of replication errors (13, 54), whereas defects in cohesin and possibly condensin genes result in reduced rates of DNA repair (29, 55), both of which might result in higher steady state levels of DNA damage; in both cases, misrepair results in GCRs. The third class was those in which defects cause increased GCR rates but no increase in Hug1 expression. It is likely that defects in these latter genes either cause defects in the DNA damage response (e.g., PKC1) (62) or defects in DNA repair that result in increased GCRs without increases in the steady state levels of DNA damage.
Previous studies have suggested that defects in the human homologs of nonessential GIS genes are prevalent in cancers that have increased genome instability (11, 63). Here, by analyzing TCGA data for 16 cancers, we identified 9 cancers with significant enrichment for LOF mutations and/or LOF plus missense mutations in the eGIS genes and 1 cancer (BRCA) in which there was only enrichment for mutations in the cohesin complex-encoding genes. Of the 9 cancers with enrichment for mutations in the eGIS genes, in 2 cases (GBM and LAML), this enrichment was only a result of the inclusion of the cohesion genes, whereas in the other 7 cancers, the enrichment was not attributable to any specific subset of eGIS genes. Furthermore, among the 9 cancers with general enrichment for mutations in the eGIS genes were 4 cancers with enrichment for mutations in chromatin remodeling/modification genes, 6 cancers with enrichment for mutations in cohesin genes, 2 cancers with enrichment for mutations in condensin genes, 1 cancer with enrichment for mutations in Smc5-Smc6 cohesin genes, and 4 cancers with enrichment for mutations in replication genes. This distribution of classes of mutated genes was also reflected among the top most frequently mutated genes in the 10 cancers with some evidence for enrichment for mutations in different eGIS genes (Fig. 4B). Defects in cohesin-encoding genes and condensin-encoding genes in cancer have been reported previously, although our analysis identified mutations in additional cancers, including GBM, low-grade glioma, and UCEC (cohesin) and stomach adenocarcinoma and UCEC (condensin) beyond those in which defects have been previously reported (64). We also observed mutations in a broad diversity of genes encoding proteins that function in DNA replication beyond the more generally observed mutator mutations affecting DNA polymerases delta and epsilon, supporting the idea that DNA replication errors could contribute to increased genome instability in cancer (53). Overall, our results support the view that eGIS genes are significantly mutated in a number of cancers. The mutations observed could be reduced-function mutations that in some cases might be dominant, loss-of-function mutations that could cause haploinsufficiency, or gain-of-function dominant mutations. Additional studies will be required to determine the frequency and nature of defects in essential and nonessential GIS genes in different cancers and whether these actually cause genome instability in these cancers.
Methods
S. cerevisiae Strains.
The dGCR and sGCR query strains used for systematic mating, RDKY7635 (dGCR; MATα hom3-10 ura3Δ0 leu2Δ0 trp1Δ63 his3Δ200 lyp1::TRP1 cyh2-Q38K iYFR016C::PMFA1-LEU2 can1::PLEU2-NAT yel072w::CAN1/URA3) and RDKY7964 (sGCR; MATα hom3–10 ura3Δ0 leu2Δ0 trp1Δ63 his3Δ200 lyp1::TRP1 cyh2-Q38K iYFR016C::PMFA1-LEU2 can1::PLEU2-NAT yel068c::CAN1/URA3), were described previously (11). The query strain RDKY8174 (MATα hom3-10 ura3Δ0 leu2Δ0 trp1Δ63 his3Δ200 lyp1::TRP1 cyh2-Q38K iYFR016C::PMFA1-LEU2 can1::PLEU2-NAT yel072w::CAN1/URA3 HUG1-EGFP.hphNT1) was used to introduce the HUG1-GFP reporter.
Systematic Strain Construction and Screening.
The dGCR and sGCR query strains were crossed to selected strains from the tsv6 temperature-sensitive mutant collection (BY4741 MATa strains; obtained from Charles Boone, Donnelly Centre, University of Toronto, Toronto, ON, Canada), using a RoTor instrument (Singer). Systematic mating, sporulation, haploid selection, GCR patch tests, and GCR rates were performed as described (11), except the crosses were performed at 25 °C and GCR patch tests and rates were performed at 25 °C and/or 30 °C, as indicated. To verify expected mutations in strains of interest, ∼1-kb overlapping fragments spanning the genes of interest were amplified from 2 independent colonies from the cross progeny and subjected to Sanger sequencing at a commercial facility.
Measurement of Hug1-GFP Levels.
Hug1-GFP abundance in log phase cells was measured at 25 °C and 30 °C using FACS, as described (52), using the following modifications: Cells were grown and processed in 96-well plates and analyzed using a BD LSR Fortessa analytical cytometer with an HTS loader. Excitation was at 488 nm, and the fluorescence signal was collected through a 505-nm long-pass filter and a HQ510/20 band-pass filter (Chroma Technology Corp). For each sample, 30,000 events were recorded. The mean value of GFP abundance was calculated using FlowJo software and normalized to the mean GFP value in wild-type cells.
Analysis of Cancer Genomics Data.
TCGA data were obtained from the cBIO portal (http://www.cbioportal.org). Simulations to determine statistical significance were performed as described (SI Appendix, Methods) (11). Prediction of the functional impact of missense mutations was determined as described (SI Appendix, Methods) (11), using 9 prediction tests (Datasets S4–S6); to be predicted deleterious, a missense mutation had to be scored as deleterious in at least 5 tests (Ndamage ≥ 5), as this cutoff captured known recurrent missense mutations in POLE, FBXW7, KAT8, MTOR, and SETX.
Supplementary Material
Acknowledgments
We thank Dr. Charlie Boone for the ts mutants. This work was supported by NIH grant R01GM26017 (to R.D.K.), Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (Brazil) grant (23038.004629/2014-19 to S.J.d.S.), and the Ludwig Institute (R.D.K., C.D.P., and S.J.d.S.).
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1906921116/-/DCSupplemental.
References
- 1.Loeb L. A., A mutator phenotype in cancer. Cancer Res. 61, 3230–3239 (2001). [PubMed] [Google Scholar]
- 2.Vogelstein B., et al. , Cancer genome landscapes. Science 339, 1546–1558 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nik-Zainal S., et al. ; Breast Cancer Working Group of the International Cancer Genome Consortium , Mutational processes molding the genomes of 21 breast cancers. Cell 149, 979–993 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Palles C., et al. ; CORGI Consortium ; WGS500 Consortium , Germline mutations affecting the proofreading domains of POLE and POLD1 predispose to colorectal adenomas and carcinomas. Nat. Genet. 45, 136–144 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.de la Chapelle A., Genetic predisposition to colorectal cancer. Nat. Rev. Cancer 4, 769–780 (2004). [DOI] [PubMed] [Google Scholar]
- 6.Loeb L. A., Harris C. C., Advances in chemical carcinogenesis: A historical review and prospective. Cancer Res. 68, 6863–6872 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Inaki K., Liu E. T., Structural mutations in cancer: Mechanistic and functional insights. Trends Genet. 28, 550–559 (2012). [DOI] [PubMed] [Google Scholar]
- 8.Sansregret L., Swanton C., The role of aneuploidy in cancer evolution. Cold Spring Harb. Perspect. Med. 7, a028373 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.D’Andrea A. D., Susceptibility pathways in Fanconi’s anemia and breast cancer. N. Engl. J. Med. 362, 1909–1919 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kobayashi H., Ohno S., Sasaki Y., Matsuura M., Hereditary breast and ovarian cancer susceptibility genes (review). Oncol. Rep. 30, 1019–1029 (2013). [DOI] [PubMed] [Google Scholar]
- 11.Putnam C. D., et al. , A genetic network that suppresses genome rearrangements in Saccharomyces cerevisiae and contains defects in cancers. Nat. Commun. 7, 11256 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Putnam C. D., Kolodner R. D., Pathways and mechanisms that prevent genome instability in Saccharomyces cerevisiae. Genetics 206, 1187–1225 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen C., Kolodner R. D., Gross chromosomal rearrangements in Saccharomyces cerevisiae replication and recombination defective mutants. Nat. Genet. 23, 81–85 (1999). [DOI] [PubMed] [Google Scholar]
- 14.Chan J. E., Kolodner R. D., A genetic and structural study of genome rearrangements mediated by high copy repeat Ty1 elements. PLoS Genet. 7, e1002089 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Putnam C. D., Hayes T. K., Kolodner R. D., Specific pathways prevent duplication-mediated genome rearrangements. Nature 460, 984–989 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kanellis P., et al. , A screen for suppressors of gross chromosomal rearrangements identifies a conserved role for PLP in preventing DNA lesions. PLoS Genet. 3, e134 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Myung K., Datta A., Kolodner R. D., Suppression of spontaneous chromosomal rearrangements by S phase checkpoint functions in Saccharomyces cerevisiae. Cell 104, 397–408 (2001). [DOI] [PubMed] [Google Scholar]
- 18.Hackett J. A., Feldser D. M., Greider C. W., Telomere dysfunction increases mutation rate and genomic instability. Cell 106, 275–286 (2001). [DOI] [PubMed] [Google Scholar]
- 19.Pennaneach V., Kolodner R. D., Stabilization of dicentric translocations through secondary rearrangements mediated by multiple mechanisms in S. cerevisiae. PLoS One 4, e6389 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Putnam C. D., Pennaneach V., Kolodner R. D., Chromosome healing through terminal deletions generated by de novo telomere additions in Saccharomyces cerevisiae. Proc. Natl. Acad. Sci. U.S.A. 101, 13262–13267 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Putnam C. D., Pennaneach V., Kolodner R. D., Saccharomyces cerevisiae as a model system to define the chromosomal instability phenotype. Mol. Cell. Biol. 25, 7226–7238 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Putnam C. D., Pallis K., Hayes T. K., Kolodner R. D., DNA repair pathway selection caused by defects in TEL1, SAE2, and de novo telomere addition generates specific chromosomal rearrangement signatures. PLoS Genet. 10, e1004277 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chan J. E., Kolodner R. D., Rapid analysis of Saccharomyces cerevisiae genome rearrangements by multiplex ligation-dependent probe amplification. PLoS Genet. 8, e1002539 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schmidt K. H., Wu J., Kolodner R. D., Control of translocations between highly diverged genes by Sgs1, the Saccharomyces cerevisiae homolog of the Bloom’s syndrome protein. Mol. Cell. Biol. 26, 5406–5420 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Myung K., Chen C., Kolodner R. D., Multiple pathways cooperate in the suppression of genome instability in Saccharomyces cerevisiae. Nature 411, 1073–1076 (2001). [DOI] [PubMed] [Google Scholar]
- 26.Putnam C. D., Hayes T. K., Kolodner R. D., Post-replication repair suppresses duplication-mediated genome instability. PLoS Genet. 6, e1000933 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Smith S., et al. , Mutator genes for suppression of gross chromosomal rearrangements identified by a genome-wide screening in Saccharomyces cerevisiae. Proc. Natl. Acad. Sci. U.S.A. 101, 9039–9044 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stirling P. C., et al. , The complete spectrum of yeast chromosome instability genes identifies candidate CIN cancer genes and functional roles for ASTRA complex components. PLoS Genet. 7, e1002057 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.De Piccoli G., et al. , Smc5-Smc6 mediate DNA double-strand-break repair by promoting sister-chromatid recombination. Nat. Cell Biol. 8, 1032–1034 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Banerjee S., et al. , Mph1p promotes gross chromosomal rearrangement through partial inhibition of homologous recombination. J. Cell Biol. 181, 1083–1093 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Motegi A., Kuntz K., Majeed A., Smith S., Myung K., Regulation of gross chromosomal rearrangements by ubiquitin and SUMO ligases in Saccharomyces cerevisiae. Mol. Cell. Biol. 26, 1424–1433 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schmidt K. H., Kolodner R. D., Suppression of spontaneous genome rearrangements in yeast DNA helicase mutants. Proc. Natl. Acad. Sci. U.S.A. 103, 18196–18201 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Huang M. E., Rio A. G., Nicolas A., Kolodner R. D., A genomewide screen in Saccharomyces cerevisiae for genes that suppress the accumulation of mutations. Proc. Natl. Acad. Sci. U.S.A. 100, 11529–11534 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Huang M. E., Kolodner R. D., A biological network in Saccharomyces cerevisiae prevents the deleterious effects of endogenous oxidative DNA damage. Mol. Cell 17, 709–720 (2005). [DOI] [PubMed] [Google Scholar]
- 35.Albuquerque C. P., et al. , Distinct SUMO ligases cooperate with Esc2 and Slx5 to suppress duplication-mediated genome rearrangements. PLoS Genet. 9, e1003670 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Putnam C. D., et al. , Bioinformatic identification of genes suppressing genome instability. Proc. Natl. Acad. Sci. U.S.A. 109, E3251–E3259 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Banerjee S., Myung K., Increased genome instability and telomere length in the elg1-deficient Saccharomyces cerevisiae mutant are regulated by S-phase checkpoints. Eukaryot. Cell 3, 1557–1566 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Myung K., Smith S., Kolodner R. D., Mitotic checkpoint function in the formation of gross chromosomal rearrangements in Saccharomyces cerevisiae. Proc. Natl. Acad. Sci. U.S.A. 101, 15980–15985 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Budd M. E., Reis C. C., Smith S., Myung K., Campbell J. L., Evidence suggesting that Pif1 helicase functions in DNA replication with the Dna2 helicase/nuclease and DNA polymerase delta. Mol. Cell. Biol. 26, 2490–2500 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hwang J. Y., et al. , Smc5-Smc6 complex suppresses gross chromosomal rearrangements mediated by break-induced replications. DNA Repair (Amst.) 7, 1426–1436 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Allen-Soltero S., Martinez S. L., Putnam C. D., Kolodner R. D., A saccharomyces cerevisiae RNase H2 interaction network functions to suppress genome instability. Mol. Cell. Biol. 34, 1521–1534 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Colosio A., Frattini C., Pellicanò G., Villa-Hernández S., Bermejo R., Nucleolytic processing of aberrant replication intermediates by an Exo1-Dna2-Sae2 axis counteracts fork collapse-driven chromosome instability. Nucleic Acids Res. 44, 10676–10690 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Deng S. K., Yin Y., Petes T. D., Symington L. S., Mre11-Sae2 and RPA collaborate to prevent palindromic gene amplification. Mol. Cell 60, 500–508 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shah K. A., et al. , Role of DNA polymerases in repeat-mediated genome instability. Cell Rep. 2, 1088–1095 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhang Y., et al. , Genome-wide screen identifies pathways that govern GAA/TTC repeat fragility and expansions in dividing and nondividing yeast cells. Mol. Cell 48, 254–265 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Weinstein J. N., et al. ; Cancer Genome Atlas Research Network , The cancer genome Atlas pan-cancer analysis project. Nat. Genet. 45, 1113–1120 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Li Z., et al. , Systematic exploration of essential yeast gene function with temperature-sensitive mutants. Nat. Biotechnol. 29, 361–367 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Huo L., et al. , The Rix1 (Ipi1p-2p-3p) complex is a critical determinant of DNA replication licensing independent of their roles in ribosome biogenesis. Cell Cycle 11, 1325–1339 (2012). [DOI] [PubMed] [Google Scholar]
- 49.Nissan T. A., et al. , A pre-ribosome with a tadpole-like structure functions in ATP-dependent maturation of 60S subunits. Mol. Cell 15, 295–301 (2004). [DOI] [PubMed] [Google Scholar]
- 50.Tanaka S., Araki H., Helicase activation and establishment of replication forks at chromosomal origins of replication. Cold Spring Harb. Perspect. Biol. 5, a010371 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Basrai M. A., Velculescu V. E., Kinzler K. W., Hieter P., NORF5/HUG1 is a component of the MEC1-mediated checkpoint response to DNA damage and replication arrest in Saccharomyces cerevisiae. Mol. Cell. Biol. 19, 7041–7049 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Srivatsan A., et al. , The Swr1 chromatin-remodeling complex prevents genome instability induced by replication fork progression defects. Nat. Commun. 9, 3680 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rayner E., et al. , A panoply of errors: Polymerase proofreading domain mutations in cancer. Nat. Rev. Cancer 16, 71–81 (2016). [DOI] [PubMed] [Google Scholar]
- 54.Lemoine F. J., Degtyareva N. P., Lobachev K., Petes T. D., Chromosomal translocations in yeast induced by low levels of DNA polymerase a model for chromosome fragile sites. Cell 120, 587–598 (2005). [DOI] [PubMed] [Google Scholar]
- 55.Jeppsson K., Kanno T., Shirahige K., Sjögren C., The maintenance of chromosome structure: Positioning and functioning of SMC complexes. Nat. Rev. Mol. Cell Biol. 15, 601–614 (2014). [DOI] [PubMed] [Google Scholar]
- 56.Ben-Aroya S., et al. , Proteasome nuclear activity affects chromosome stability by controlling the turnover of Mms22, a protein important for DNA repair. PLoS Genet. 6, e1000852 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Nie M., Boddy M. N., Cooperativity of the SUMO and ubiquitin pathways in genome stability. Biomolecules 6, 14 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gerhold C. B., Hauer M. H., Gasser S. M., INO80-C and SWR-C: Guardians of the genome. J. Mol. Biol. 427, 637–651 (2015). [DOI] [PubMed] [Google Scholar]
- 59.Loeillet S., et al. , Genetic network interactions among replication, repair and nuclear pore deficiencies in yeast. DNA Repair (Amst.) 4, 459–468 (2005). [DOI] [PubMed] [Google Scholar]
- 60.Shimada K., et al. , TORC2 signaling pathway guarantees genome stability in the face of DNA strand breaks. Mol. Cell 51, 829–839 (2013). [DOI] [PubMed] [Google Scholar]
- 61.Mischo H. E., et al. , Yeast Sen1 helicase protects the genome from transcription-associated instability. Mol. Cell 41, 21–32 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Soriano-Carot M., Quilis I., Bañó M. C., Igual J. C., Protein kinase C controls activation of the DNA integrity checkpoint. Nucleic Acids Res. 42, 7084–7095 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Knijnenburg T. A., et al. , Genomic and molecular landscape of DNA damage repair deficiency across the cancer genome Atlas. Cell Rep. 23, 239–254.e6 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Leiserson M. D., et al. , Pan-cancer network analysis identifies combinations of rare somatic mutations across pathways and protein complexes. Nat. Genet. 47, 106–114 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.