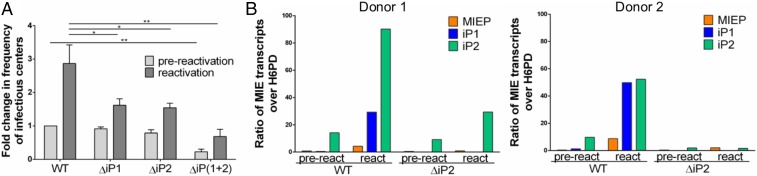
Fig. 4.
The intronic promoters are required for reactivation of HCMV from latency in CD34+ HPCs. CD34+ HPCs were infected with TB40/E WT, ΔiP1, ΔiP2, or ΔiP(1 + 2) expressing GFP as a marker for infection for 24 h (MOI = 2). Pure populations of infected (GFP+) CD34+ cells were isolated by FACS and maintained in long-term bone marrow culture for 10 d. (A) Viable CD34+ HPCs were seeded by limiting dilution onto monolayers of permissive fibroblasts in a cytokine-rich media to promote myeloid differentiation (reactivation, dark gray). An equivalent number of cells was mechanically lysed and seeded in parallel to determine the infectious virus present prior to reactivation (prereactivation, light gray). The frequency of infectious centers formed was determined 14 d later by ELDA from the fraction of GFP+ wells at each dilution. Data are expressed as fold change over the frequency of infectious centers produced by the WT virus prior to reactivation. Data from 3 independent biological replicates are shown; SE is depicted. Statistical significance was determined by multiple t tests comparing each mutant virus to the WT parental virus (*P value ≤ 0.05; **P value ≤ 0.005). (B) At day 10, RNA was collected from CD34+ HPCs latently infected with WT or ΔiP2 HCMV (prereactivation, pre). The remaining cells were plated in a modified “cell-free” reactivation assay absent coculture with permissive fibroblasts and in reactivation media enriched with 45 ng/mL of IL-6, G-CSF, and GM-CSF for 7 d before RNA was collected from adherent cells (react). RT-qPCR was performed to quantify discrete MIE transcripts relative to H6PD. Data from 2 independent biological replicates (qPCR reaction performed in triplicate) using cells from multiple donors are shown.