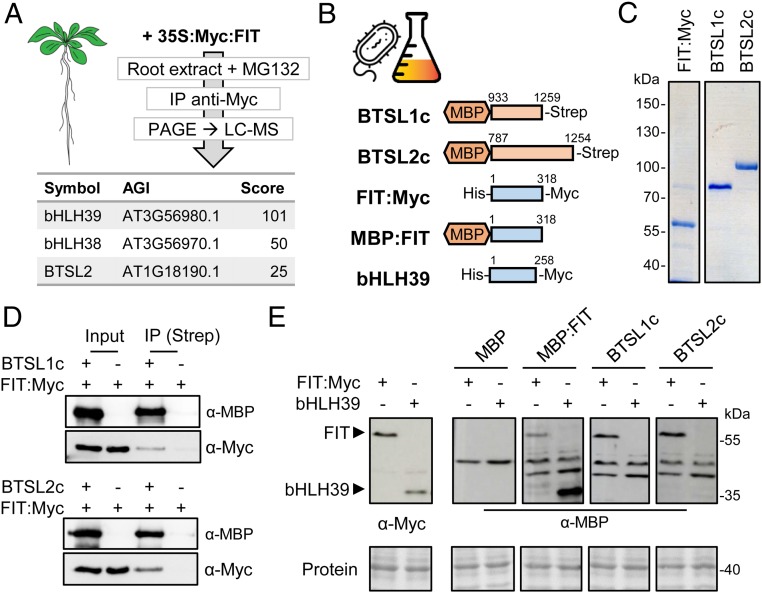
Fig. 5.
The C-terminal domain of BTSL1 or BTSL2 interacts with FIT. (A) BTSL2 coimmunoprecipitated (IP) with FIT from extracts of Arabidopsis roots. Proteins were separated by SDS/PAGE and identified by LC-MS. (B) Diagram of the recombinant proteins produced in E. coli for in vitro analyses. (C) Purified FIT, BTSL1c, and BTSL2c proteins stained with Coomassie blue. (D) Interaction between the C-terminal E3 ligase domain of BTSL proteins and FIT shown by immunoprecipitation. (E) Far-Western blot analysis to compare the interaction of BTSL proteins with FIT and its partner bHLH39. Protein extracts of bacteria producing FIT:Myc or bHLH39:Myc were separated by SDS/PAGE, blotted, and incubated with the indicated proteins, followed by immunodetection of MBP. Ponceau staining shows equal protein loading.