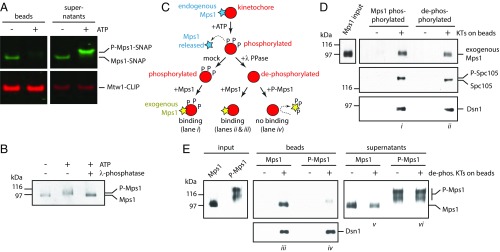
Fig. 4.
Mps1 autophosphorylation inhibits its binding to kinetochores. (A) An in vitro kinase reaction releases Mps1 from isolated kinetochores. Kinetochores carrying Mps1-SNAP 549 (green) and Mtw1-CLIP 647 (red) (from SBY15285) were immunoprecipitated on magnetic beads and incubated with or without ATP. Proteins retained on beads and released into the supernatants were analyzed by SDS/PAGE and fluorescence imaging. (B) The electrophoretic mobility shift caused by incubation of Mps1 with ATP is due to phosphorylation. Mps1 was purified (from SBY12412) via immunoprecipitation under stringent conditions to remove copurifying proteins and then incubated with or without ATP. An aliquot of the ATP-treated sample was subsequently treated with λ-phosphatase. The resulting samples were analyzed by immunoblotting. (C) Schematic illustrating the preparation of Mps1-phosphorylated and dephosphorylated kinetochores for binding to exogenous Mps1. (D) Mps1 binds equivalently to Mps1-phosphorylated or dephosphorylated kinetochores. Kinetochores (from SBY9190) and control beads lacking kinetochores were prepared, incubated with purified exogenous Mps1 (from SBY12412, as diagrammed in C), washed, and then analyzed by immunoblotting. (E) Autophosphorylated Mps1 fails to bind kinetochores. Immobilized kinetochores (from SBY9190) and beads lacking kinetochores were treated sequentially with ATP and then λ-phosphatase to release endogenous Mps1 and remove phosphates. They were then washed and incubated with native Mps1 or with autophosphorylated P-Mps1 (from SBY12412, as diagrammed in C). Native Mps1 that had not been autophosphorylated in vitro bound well to the kinetochores (lane iii), with some excess remaining in the supernatant (lane v). Autophosphorylated P-Mps1 bound poorly (lane iv). Electrophoretic mobility of the minor, kinetochore-bound P-Mps1 subfraction (lane iv) was faster than that of the major unbound fraction (lane vi), indicating that kinetochores selectively bind less-phosphorylated forms of Mps1.