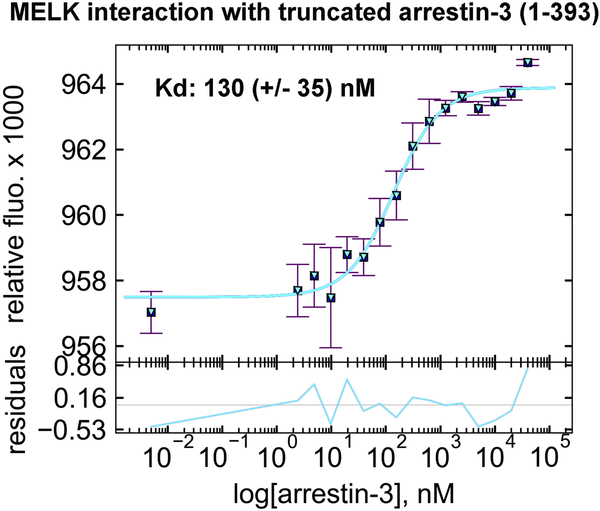
Figure 2. The affinity of MELK1−326,T167E for arrestin-31−393 in the presence of ATP.
A binding curve for bovine arrestin-31−393 with human MELK1−326,T167E in the presence of 1 mM ATP and 2 mM MgCl2 is shown with the average Kd value (n=3). Microscale thermophoresis was performed at a constant concentration of Tris-NTA-labeled His-MELK1−326,T167E (50 nM) with a serial dilution of arrestin-31−393 (0–40 μM). The binding isotherm was calculated using preset T-jump in the software PALMIST [59, 60], and the graph was created in the program GUSSI.