Genes encoding spliceosome components including SF3B1, U2AF1, and SRSF2, are frequently somatically mutated in myelodysplastic syndromes (MDS), other hematologic malignancies, and solid tumors.1 Typically these are mutually exclusive, heterozygous, missense, hotspot mutations that result in neomorphic or gain-of-function splicing phenotypes. These mutations alter splicing of many genes; however, overlap among the different splicing factors is limited. Thus, a common mechanism by which spliceosome mutations contribute to disease is suspected but has remained elusive.
We previously demonstrated that inhibiting any of five different spliceosome genes (SF3B1, SF3A1, SF3A2, SF3A3, EFTUD2) in mouse or human macrophages reduces inflammatory cytokine production induced by multiple Toll-like receptor (TLR) agonists including the TLR4 agonist lipopolysaccharide (LPS).2–5 Although these genes encode essential spliceosome components, partial gene knockdown (approx. 80%) reduces LPS-induced inflammatory cytokine production without affecting viability or phagocytosis.2–5 Hence, innate immunity may be particularly sensitive to spliceosome perturbation.
These observations suggest that MDS-associated spliceosome mutations might enhance innate immunity, which could contribute to disease pathogenesis.6 Inflammatory cytokines are increased in serum and bone marrow of MDS patients, and increased cytokine levels correlate with a worse outcome.6 Here we show that mutations in SF3B1, U2AF1, and SRSF2 enhance NFκB activity and LPS-induced inflammatory cytokine production in macrophages, patient-derived cell lines, and mouse and human myeloid cells. These changes could represent a common mechanism that contributes to MDS pathogenesis.
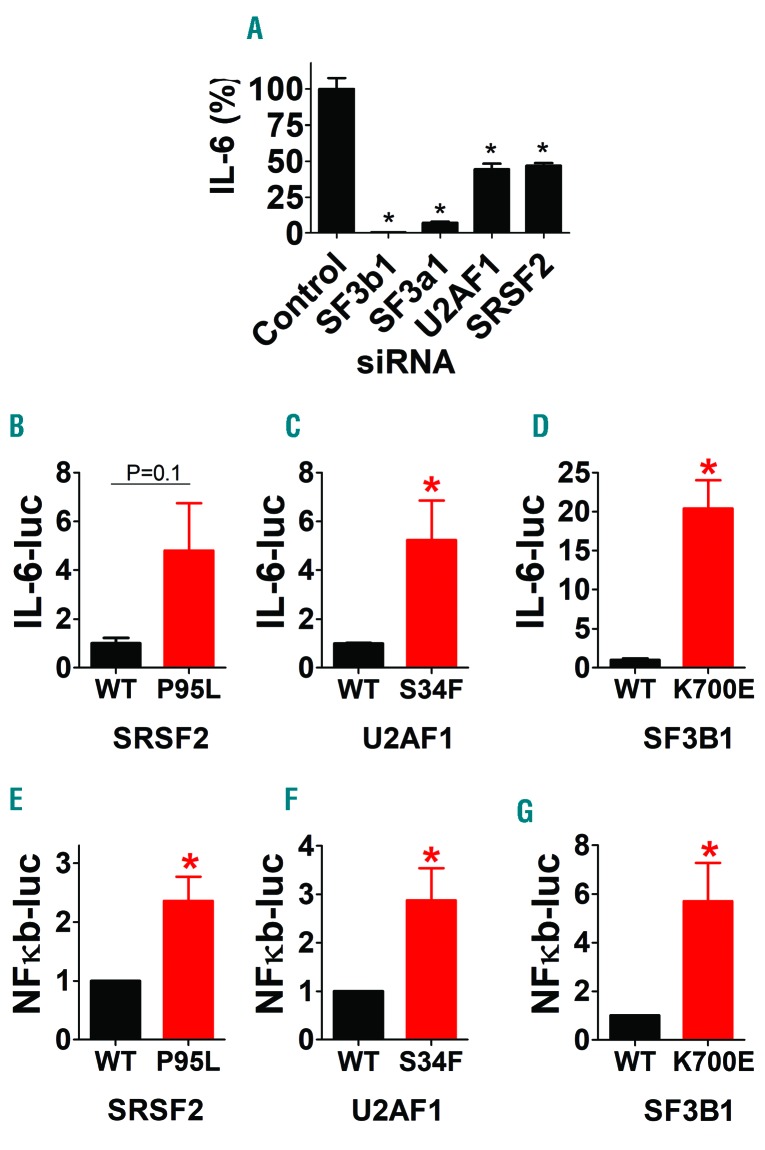
We previously demonstrated that inhibition of SF3A1 or SF3B1 in macrophages reduces LPS-induced inflammatory cytokine production.2 To determine if this finding extends to all three commonly mutated spliceosome genes, we additionally inhibited U2af1 and Srsf2 in RAW264.7 mouse macrophages using RNAi, challenged the cells with LPS, and monitored IL-6 production. Inhibition of any of the four spliceosome genes reduced LPS-induced IL-6 production (Figure 1A) without significantly affecting viability (data not shown). Thus, these spliceosome genes exert a common effect on LPS-induced cytokine production.
Figure 1.
Spliceosome gene mutations found in myelodysplastic syndrome (MDS) patients enhance innate immunity signaling in macrophages. (A) siRNA targeting the indicated spliceosome genes or a control non-targeting siRNA were transfected into RAW264.7 mouse macrophages, the cells were then stimulated with 20 ng/mL lipopolysaccharide (LPS) for 6 hours (h), and IL-6 protein production was monitored by ELISA. (B-G) Plasmids expressing the indicated wild-type (WT) (black) or mutant (red) spliceosome genes were co-transfected into RAW264.7 cells along with either an IL-6 luciferase reporter construct (B-D) or an NFκB-dependent luciferase reporter construct (E-G) and a SV40-rluc construct for normalization. The cells were then stimulated with 20 ng/mL LPS for 6 h, and luciferase activity was monitored. Data represent mean, Standard Error of Mean. *P<0.05. If no P-value is indicated, the comparison was not significantly different.
Because spliceosome inhibition reduced inflammatory cytokine levels, we assessed if MDS-associated spliceosome mutations would have the converse effect. We co-transfected into RAW264.7 macrophages either wild-type (U2AF1-WT, SRSF2-WT, or SF3B1-WT) or mutant (U2AF1-S34F, SRSF2-P95L, or SF3B1-K700E) spliceosome genes, an IL-6-luciferase reporter, and an SV40-rluc normalization reporter. We then stimulated the cells with LPS and monitored IL-6-luciferase production. All three mutant spliceosome genes enhanced IL-6-luciferase production compared to wild-type spliceosome genes (Figure 1B-D). To determine what drives this increased IL-6, we assessed NFκB activation using an NFκB-luciferase reporter. All three mutant spliceosome genes enhanced NFκB-luciferase production compared to the wild-type genes (Figure 1E-G).
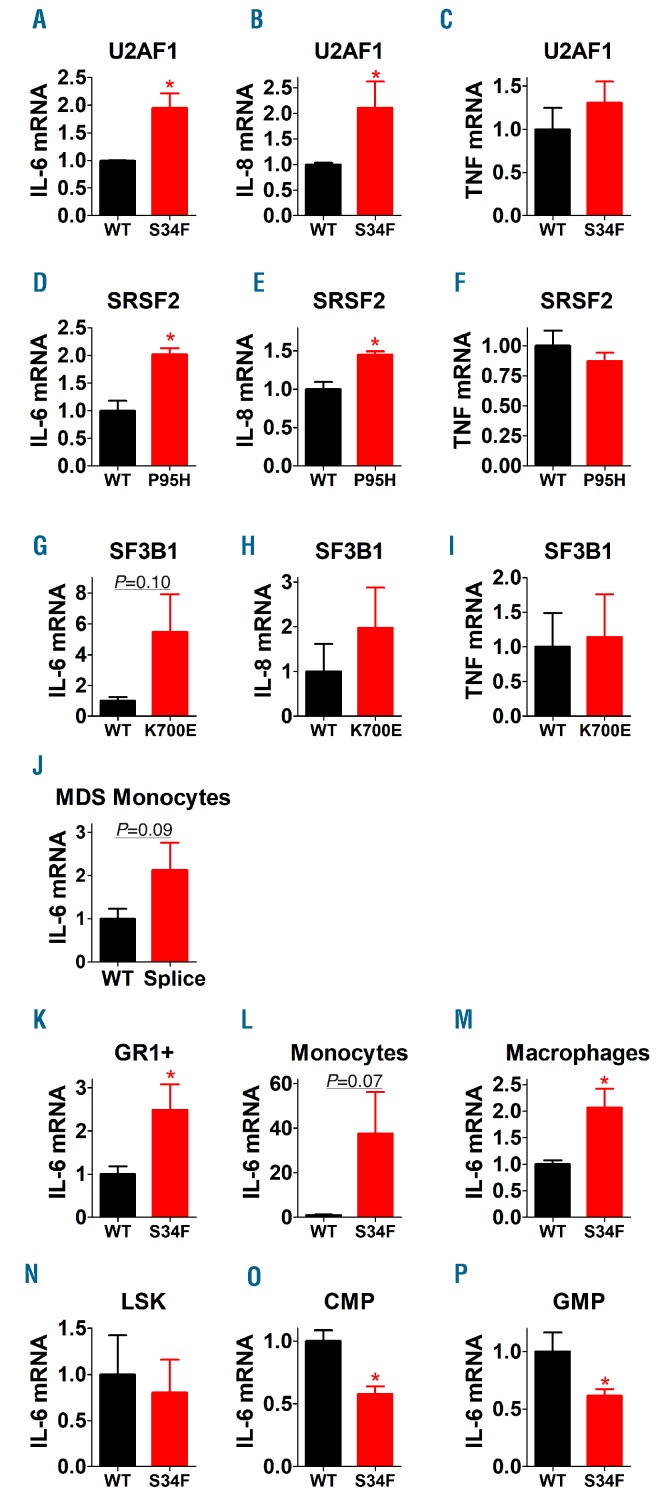
To determine if spliceosome mutations enhanced inflammatory cytokine production in myeloid malignancy-relevant cells, we examined the effect of these mutations when engineered into the K562 myeloid leukemia line. The U2AF1-S34F mutant line had higher basal levels of IL-6 and IL-8, but not TNFα, mRNA compared to the U2AF1-wt line (Figure 2A-C). Cells with mutated SRSF2-P95H likewise had higher levels of IL-6 and IL-8 but not TNFα mRNA compared to the SRSF2-wt line (Figure 2D-F). Lines carrying the SF3B1-K700E mutation also had higher levels of IL-6 mRNA (Figure 2G-I), although this did not reach statistical significance, likely due to the fact that the SF3B1-K700E mutation was not stable in the K562 line and rapidly reverted to wild type. We validated these results at the protein level in SRSF2-P95H and U2AF1-S34F cells, which produced higher basal levels of IL-6 protein than control lines (Online Supplementary Figure S1). Altogether, these K562 studies indicate that increased inflammatory cytokine production is a common consequence of MDS-associated spliceosome mutations.
Figure 2.
Spliceosome gene mutations found in myelodysplastic syndrome (MDS) patients enhance inflammatory cytokine production in cancer-relevant myeloid cells. (A-I) Cytokine mRNA levels were assayed in K562 cells carrying the indicated spliceosome gene mutations (red) or wild-type (WT) control genes (black). (J) IL-6 mRNA was analyzed in lipopolysaccharide (LPS)-induced (20 ng/mL LPS for 4 hours) peripheral blood monocytes isolated from MDS patients either carrying mutations in spliceosome genes (all splice, n=8, 3 SF3B1, 3 SRSF2, 2 U2AF1, color-coded red) or not carrying mutations in these three spliceosome genes (WT, color-coded black). Patient demographics are described in the Online Supplementary Table S1. (K-P) IL-6 mRNA was analyzed in the indicated myeloid cell types from mice engineered to express either WT human U2AF1 (black) or U2AF1-S34F (red). Data represent mean, Standard Error of Mean. *P<0.05. If no P-value is indicated, the comparison was not significantly different.
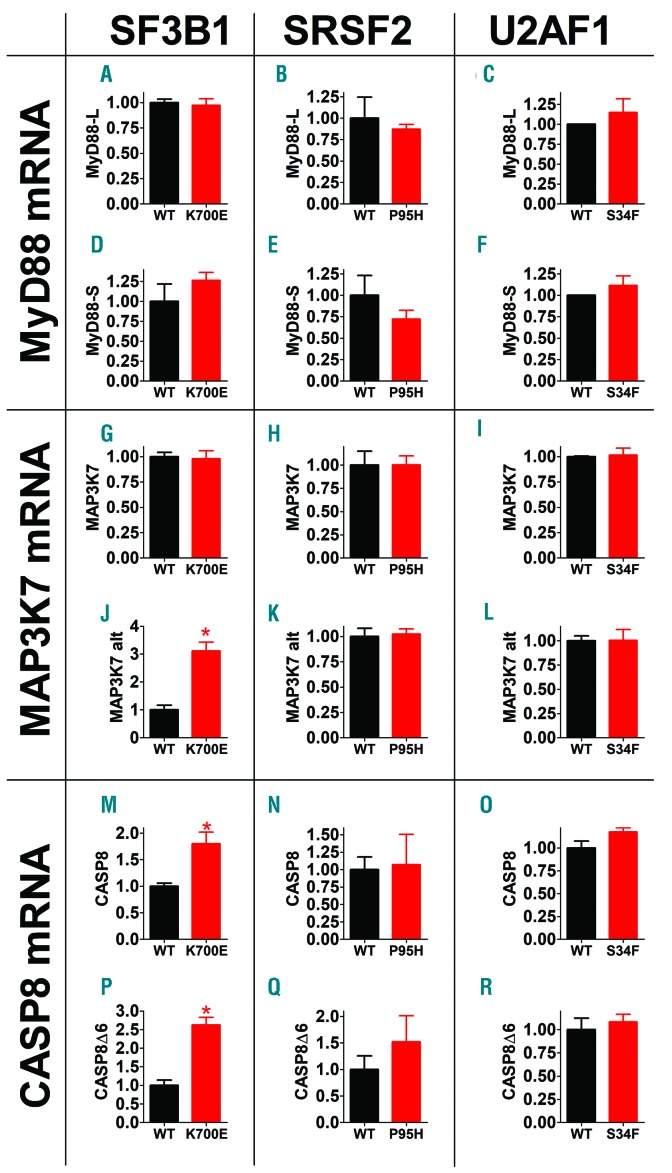
Our prior studies indicated that inhibiting SF3A1 altered splicing of many genes in the TLR signaling pathway,5 including the signaling adaptor MyD88. The MyD88 gene encodes two isoforms: a long isoform (MyD88l, an NFκB activator), and a shorter isoform in which exon two is skipped (MyD88s, an NFκB inhibitor).2 SF3a1 or SF3b1 knockdown led to increased production of anti-inflammatory MyD88s, leading to decreased inflammatory cytokine production.2 Interestingly, U2af1 or Srsf2 knockdown in RAW264.7 macrophages also increased MyD88s mRNA levels without altering MyD88l (Online Supplementary Figure S2). However, none of the three spliceosome mutations altered MyD88 splicing in K562 cells (Figure 3A-F), suggesting that altered splicing of other TLR pathway genes drives the enhanced inflammatory cytokine production in these cells.
Figure 3.
Myelodysplastic syndrome (MDS)-associated spliceosome gene mutations alter splicing of genes that regulate innate immune signaling. The indicated mRNA isoforms were monitored by isoform-specific quantitative polymerase chain reaction on RNA isolated from K562 cells carrying the indicated spliceosome gene mutations (red) or wild-type (WT) control genes (black). MAP3K7 alt refers to the alternative isoform of MAP3K7 that uses an alternate 3’ splice site in intron 4. CASP8Δ6 refers to the alternate CASP8 isoform in which exon 6 is skipped. Data represent mean, Standard Error of Mean. *P<0.05. If no P-value is indicated, the comparison was not significantly different.
Lee et al.7 reported that SF3B1 mutation altered splicing of MAP3K7, which encodes TAK1. Depending on the physiological context, TAK1 can act as either a positive or negative regulator of NFκB activation,7 and the authors found that increased production of the alternate MAP3K7 isoform correlated with enhanced innate immune signaling.7 Similarly, the authors determined that cells carrying mutations in SRSF2 produced an alternate isoform of CASPASE8 (CASP8) in which exon 6 is skipped, resulting in production of a truncated protein that enhances NFκB activity.7
To determine if altered splicing of MAP3K7 or CASP8 was associated with enhanced innate immune signaling, we monitored production of these splice forms. In K562 cells, SF3B1-K700E but not SRSF2-P95H or U2AF1-S34F mutations enhanced production of the alternate isoform of MAP3K7 (Figure 3J-L); in contrast, the canonical isoform of MAP3K7 was not affected by any spliceosome mutation (Figure 3G-I). The comparable mouse MAP3K7 alternate isoform trended towards a moderate increase in RAW264.7 cells over-expressing SF3B1-K700E compared to SF3B1-wt (Online Supplementary Figure S3).
Production of the alternate isoform of CASP8 was moderately increased in K562 cells with the SRSF2-P95H mutation, but this difference did not reach statistical significance (Figure 3Q). Nor did U2AF1-S34F mutation alter production of either CASP8 isoform (Figure 3O and R). In contrast, SF3B1-K700E mutation increased production of both CASP8 isoforms (Figure 3M and P), reflecting a possible change in expression rather than splicing. Consistent with this, two other CASP8 isoforms previously reported to be affected by spliceosome mutations7,8 were also increased in SF3B1-K700E cells (Online Supplementary Figure S4). Collectively, these data indicate that multiple alternative splicing events, including many yet to be elucidated, contribute to enhanced innate immune signaling in cells carrying spliceosome mutations.
To determine if spliceosome mutations enhanced inflammatory cytokine production in patient samples, we reanalyzed data from our prior peripheral blood mono-cyte study.9 Indeed, monocytes from MDS patients with spliceosome mutations trended towards increased IL-6 mRNA levels (Figure 2J).
To further explore the effect of spliceosome mutations on primary myeloid lineage cells, we used mice engineered to inducibly express either wild-type U2AF1 or U2AF1-S34F. Five days after induction of the U2AF1 transgene, we isolated total Gr-1-positive (+) myeloid cells and CD115+ monocytes and measured IL6 expression. As expected, IL6 expression was significantly increased in these populations, as well as in primary bone marrow-derived macrophages (Figure 2K-M). Since these spliceosome mutations are present throughout hematopoietic ontogeny, we assessed whether IL6 expression also was increased in hematopoietic stem and progenitor cells (HSPC). Interestingly, IL6 was unchanged or even slightly decreased in immature HSC-enriched U2AF1-S34F Lin−cKit+Sca-1+ (LSK) cells, lineage-committed common myeloid progenitors (CMP), and granulocyte-macrophage progenitors (GMP) (Figure 2N-P). These data suggest that increased IL6 expression is a unique property restricted to mature myeloid cells carrying spliceosome mutations.
These studies demonstrate that enhanced inflammatory cytokine production is a common consequence of MDS-associated spliceosome mutations. Consistent with this, the IL-6 pathway was altered by all three spliceosome mutations in a RASL-seq study.10 Moreover, a recent study demonstrated that SF3B1 and SRSF2 mutations enhance NFκB activity;7 our study confirms these results and extends them to U2AF1. Finally, spliceosome mutations lead to activation of the NLRP3 inflammasome,11 which also regulates inflammation.
Our studies demonstrating that innate immune signaling genes are particularly sensitive to spliceosome perturbation2–5 have led us to hypothesize that TLR signaling pathway splice site choices evolved to be exquisitely sensitive to cellular conditions because of their functional significance and that they are key regulatory points that limit inflammation.5 We speculate that altered splicing of many genes including MAP3K7 and CASP8 contributes to the enhanced innate immune signaling induced by spliceosome mutations. While knockdown of spliceosome genes weakens innate immunity, expression of mutant spliceosome genes induced the opposite phenotype. This is consistent with studies indicating that these mutations are largely neomorphic rather than loss-of-function.12,13
Increased inflammation in MDS may be a common feature that is not limited to patients with spliceosome mutations. Increased inflammation is thought to impair normal hematopoiesis and contribute to the pathogenesis by altering HSPC survival, proliferation, differentiation, and apoptosis.14 Inflammation induced by spliceosome mutations could influence malignancy development in two ways. First, myeloid cells over-producing inflammatory cytokines in the bone marrow could impair HSC niche function, leading to suppression of normal hematopoiesis.14 Innate immune cells present in the HSC niche can alter HSC function.15 In particular, IL-6 influences HSC development and has paracrine function in HSPC, leading to overproduction of myeloid cells and aberrant hematologic features found in MDS patients.16–18 Second, our results raise the possibility that altered innate immune signaling induced by spliceosome mutations could alter immune cell function, contributing to the increased risk of infection and/or hyperinflammatory features common to MDS patients,19,20 a possibility that we will explore in future studies. Finally, while we focus on the role of spliceosome mutations in MDS, spliceosome mutations are present in many hematologic malignancies1 and could affect pathogenesis of other cancers using similar mechanisms.
Acknowledgments
Thanks to Matthew Meyerson, Cara Lunn Shirai, and Peter Sporn for providing assistance and reagents.
Footnotes
Funding: this study was supported by National Institutes of Health grants R01ES025161 (to SA), K01DK098315 (to EMP), F31HL138754 (to JLR), the V Foundation Scholar Award (to RB) as well as grants from the Cancer League of Colorado, the University of Colorado Hematological Malignancies Pilot Grant Program, University of Colorado RNA Biosciences Initiative Project Grant, and the Wendy Siegel Fund for Leukemia and Cancer Research. CTJ is generously supported by the Nancy Carroll Allen Chair in Hematology Research. DAP is a Leukemia and Lymphoma Society Scholar in Clinical Research.
Information on authorship, contributions, and financial & other disclosures was provided by the authors and is available with the online version of this article at www.haematologica.org.
References
- 1.Scott LM, Rebel VI. Acquired mutations that affect pre-mRNA splicing in hematologic malignancies and solid tumors. J Natl Cancer Inst. 2013;105(20):1540–1549. [DOI] [PubMed] [Google Scholar]
- 2.De Arras L, Alper S. Limiting of the innate immune response by SF3A-dependent control of MyD88 alternative mRNA splicing. PLoS Genet. 2013;9(10):e1003855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.De Arras L, Laws R, Leach SM, et al. Comparative genomics RNAi screen identifies Eftud2 as a novel regulator of innate immunity. Genetics. 2014;197(2):485–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.De Arras L, Seng A, Lackford B, et al. An evolutionarily conserved innate immunity protein interaction network. J Biol Chem. 2013;288(3):1967–1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.O’Connor BP, Danhorn T, De Arras L, et al. Regulation of toll-like receptor signaling by the SF3a mRNA splicing complex. PLoS Genet. 2015;11(2):e1004932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Starczynowski DT, Karsan A. Innate immune signaling in the myelodysplastic syndromes. Hematol Oncol Clin North Am. 2010;24(2):343–359. [DOI] [PubMed] [Google Scholar]
- 7.Lee SC, North K, Kim E, et al. Synthetic Lethal and Convergent Biological Effects of Cancer-Associated Spliceosomal Gene Mutations. Cancer Cell. 2018;34(2):225–241 e228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ilagan JO, Ramakrishnan A, Hayes B, et al. U2AF1 mutations alter splice site recognition in hematological malignancies. Genome Research. 2015;25(1):14–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pollyea DA, Hedin BR, O’Connor BP, Alper S. Monocyte function in patients with myelodysplastic syndrome. J Leukoc Biol. 2018;104(3):641–647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Qiu J, Zhou B, Thol F, et al. Distinct splicing signatures affect converged pathways in myelodysplastic syndrome patients carrying mutations in different splicing regulators. RNA. 2016;22(10):1535–1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Basiorka AA, McGraw KL, Eksioglu EA, et al. The NLRP3 inflammasome functions as a driver of the myelodysplastic syndrome phenotype. Blood. 2016;128(25):2960–2975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dvinge H, Kim E, Abdel-Wahab O, Bradley RK. RNA splicing factors as oncoproteins and tumour suppressors. Nat Rev Cancer. 2016;16(7):413–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Saez B, Walter MJ, Graubert TA. Splicing factor gene mutations in hematologic malignancies. Blood. 2017;129(10):1260–1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Baldridge MT, King KY, Goodell MA. Inflammatory signals regulate hematopoietic stem cells. Trends Immunol. 2011;32(2):57–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Riether C, Schurch CM, Ochsenbein AF. Regulation of hematopoietic and leukemic stem cells by the immune system. Cell Death Differ. 2015;22(2):187–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Burger R. Impact of interleukin-6 in hematological malignancies. Transfusion Medicine and Hemotherapy : offizielles Organ der Deutschen Gesellschaft fur Transfusionsmedizin und Immunhamatologie. 2013;40(5):336–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Reynaud D, Pietras E, Barry-Holson K, et al. IL-6 controls leukemic multipotent progenitor cell fate and contributes to chronic myelogenous leukemia development. Cancer Cell. 2011;20(5):661–673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schipperus MR, Sonneveld P, Lindemans J, van Lom K, Vlastuin M, Abels J. Interleukin-6 and interleukin-1 enhancement of GM-CSF-dependent proliferation of haematopoietic progenitor cells in myelodysplastic syndromes. Brit J Haematol. 1991;77(4):515–522. [DOI] [PubMed] [Google Scholar]
- 19.Grignano E, Jachiet V, Fenaux P, Ades L, Fain O, Mekinian A. Autoimmune manifestations associated with myelodysplastic syndromes. Ann Hematol. 2018;97(11):2015–2023. [DOI] [PubMed] [Google Scholar]
- 20.Pagano L, Caira M. Risks for infection in patients with myelodysplasia and acute leukemia. Curr Opin Infect Dis. 2012;25(6):612–618. [DOI] [PubMed] [Google Scholar]