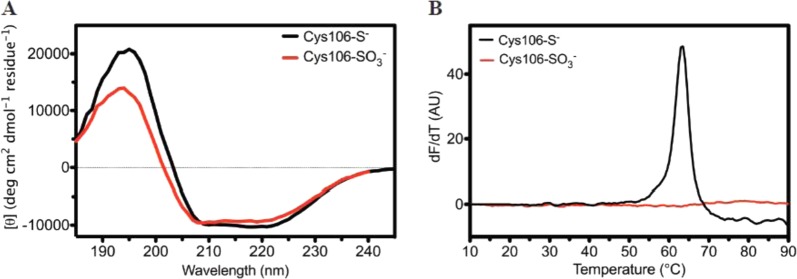
Fig. 5. Cys106-SO3− DJ-1 formation preserves secondary structure but destabilizes the protein.
a Circular dichroism (CD) spectra are shown for reduced (black) and Cys106-SO3− DJ-1 (red) DJ-1. Formation of Cys106-SO3− results in a minor decrease in secondary structural content. The y-axis shows per-residue molar ellipticity ([θ]). b The Thermofluor scanning fluorimetry assay was used to measure the melting temperature (Tm) of reduced (black) and Cys106-SO3− (red) DJ-1. The first derivative of the fluorescence as a function of temperature (dF/dT) is shown. Reduced DJ-1 has a Tm of 63 °C, while there is no measured transition for Cys106-SO3− DJ-1, indicating substantial destabilization