Abstract
Aims/Introduction
There is potential for mobile applications to deliver new self‐management interventions for chronic disease, especially in diabetes. The aim of the present study was to evaluate the effects of a mobile phone application (MPA) combined with or without self‐monitoring of blood glucose (SMBG) on glycemic control in patients with diabetes.
Materials and Methods
The study was a 24‐week period, four‐arm parallel group, non‐blinded, randomized trial. A total of 185 patients with mean age of 52 years were randomized to group A (no MPA and no SMBG), group B (SMBG only), group C (MPA only) and group D (both MPA and SMBG were used). Changes in glycated hemoglobin (HbA1c), fasting plasma glucose and 1,5‐anhydroglucitol from baseline to week 24 were analyzed.
Results
At 24 weeks, the HbA1c levels in patients of all groups decreased significantly from baseline. There were significant differences in the proportions of patients that achieved HbA1c <7% between groups, especially in group C and group D, compared with group A at week 24 (60.4%, 62.2% vs 25.5%, all P < 0.05). 1,5‐Anhydroglucitol changes were obvious in group A and group C at week 24 from baseline (all P < 0.05 within groups). Factorial analysis of anova showed that MPA intervention was the main effective factor for HbA1c change (F = 4.59, P = 0.034), and there was no effect on HbA1c change for SMBG intervention (P = 0.975).
Conclusions
Implementation of the MPA, Diabetes‐Carer, is effective in improving the proportion of HbA1c <7% in patients with type 2 diabetes.
Keywords: Diabetes mellitus, Mobile applications, Self‐monitoring of blood glucose
Introduction
Diabetes mellitus, which is characterized by a persistent metabolic disorder of blood glucose, protein and fat, is an increasing epidemic world1. The International Diabetes Federation has approached an extrapolation of 592 million adults suffering from diabetes in 2035 based on data from 219 countries2. However, in an updated national investigation of Chinese adults in 2013, the overall prevalence of diabetes was 10.9%, and for prediabetes 35.7%, implying that up to nearly half of the population already had impaired blood glucose3. In addition, diabetes leads to a high mortality due to its various distant complications, including macrovascular diseases, such as coronary artery disease and stroke, as well as microvascular disease, such as nephropathy and retinopathy1, 3. These facts confirm the severity and criticality of diabetes as a health problem in the world, especially in low‐income countries and districts.
According to the national survey in 2013, the awareness, treatment and control rate of diabetes in the Chinese population is significantly lower than expected3. It is evident that optimal glycemic control is one of the key factors for preventing and alleviating diabetes‐related complications, which requires intensive education, effective lifestyle interventions, active self‐monitoring and a pharmacological plan to achieve4. With social development, the shortcomings of traditional self‐management interventions, such as non‐personalized education, incomplete blood glucose recording and delayed face‐to‐face communication, are leading to uncontrolled glycemic goal and serious diabetes outcomes5, 6. Nowadays, the use of new health technologies – computer‐ or mobile phone‐based self‐management – might theoretically play an important role in chronic disease management, particularly in diabetes7, 8. In 2008, WellDoc mobile diabetes management was used for the first time in diabetes patients, showing that glycemic control was significantly improved9. A systematic review and meta‐analysis has shown that smartphone‐based self‐management applications have moderate benefits on not only the reduction of the glycated hemoglobin level (−0.4%), but also the enhancement of diabetes knowledge and improvement of lifestyle changes10. In addition, numerous studies have also shown that utilizing smartphone applications for diabetes might help to improve the clinical outcomes6, 11, 12. Anyway, the popularity of smartphones allows them to be a convenient and effective platform for healthcare delivery.
To date, the effectiveness of mobile phone applications (MPA) for diabetes has not been fully assessed in a Chinese population. In the present study, we presented a MPA called “Diabetes‐Carer,” which provides several functions, such as medical history recording, real‐time feedback coaching and blood glucose analyzing, as a management platform for type 2 diabetes patients. The objective of the present study was to estimate the impact of Diabetes‐Carer combined with or without self‐monitoring of blood glucose (SMBG) on glycemic control and other clinical outcomes for type 2 diabetes patients in 24 weeks.
Methods
Trial design
The study was a 24‐week period, four‐arm parallel group, non‐blinded, randomized trial (Clinicaltrials.gov ChiCTR‐IOR‐16008638). Participants were recruited from the outpatient department of Shanghai East Hospital during June 2016 to January 2017. Using the block randomization method, participants were randomly divided into four intervention groups in 1:1:1:1 ratios: group A (no MPA and no SMBG), group B (SMBG only), group C (MPA only) and group D (both MPA and SMBG were used; Figure 1). The randomization sequence was created by Stata software version 12.0 (StataCorp, College Station, TX, USA). One staff not associated with the clinical work of the study generated the randomization sequence and prepared the sequentially numbered opaque envelopes independently.
Figure 1.
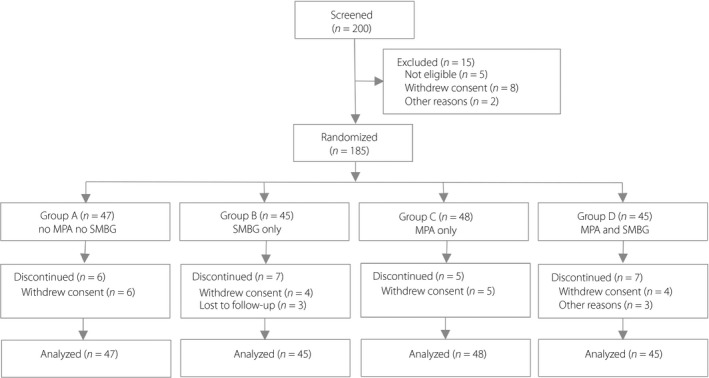
Study design and participant flow diagram for the present study. MPA, mobile phone application; SMBG, self‐monitoring of blood glucose.
Participants
Inclusion criteria were: (i) men or women aged 35–65 years; (ii) diagnosed with type 2 diabetes according to 1999 World Health Organization criteria; (iii) able to use a mobile phone; and (iv) signed the informed consent form. No limitation for glycated hemoglobin (HbA1c) was defined.
Exclusion criteria were: (i) patients with severe liver, kidney and systemic diseases; (ii) patients who used insulin pump; (iii) patients who were pregnant or planned to be pregnant in 6 months; (iv) excessive drinking or drug users; (v) patients with mental illness and were receiving treatment; (vi) severe visual impairment; (vii) unable to access the web; and (viii) the possibility of loss to follow up according to the judgment of the researchers.
All patients provided written informed consent before their enrollment. The study was approved by the institutional review board of Shanghai East Hospital (Approval No. 2016 (015)), and conformed to the provisions of the Declaration of Helsinki.
Interventions
In the control group (group A), patients received their usual treatment once a month and did not use a designated blood glucose meter. They did not use MPA either. Their physicians adjusted their medication regimens according to their blood glucose records when they were available.
In the SMBG groups (group B and group D), each patient received a designated blood glucose meter and an adequate number of strips. The blood glucose meter and strips were provided by Bayer Healthcare (Contour TS; Shanghai, China).
In the MPA groups (group C and group D), each patient was asked to install an application called Diabetes‐Carer on their mobile phone. The Diabetes‐Carer application, designed by Hangzhou Kang Sheng Health Management Co., Ltd. (Hangzhou, China; http://www.91jkys.com/), is a mobile phone‐based diabetes management platform, which can be used by both patients and clinicians. Diabetes‐Carer for patients consists of four main parts: diabetic education, self‐management, patient community, and real‐time communication between patients and clinicians.
In the diabetic education component, the MPA teaches patients to adopt a better lifestyle through a combination of these interventions: (i) establishing a diet library, which provided descriptions of the glycemic index for each food item, analysis of nutrition ingredients and calculation of energy intake; (ii) video demonstration of special anti‐resistance sports, and picture demonstration of usual exercise to motivate self‐care activities; and (iii) knowledge and information about blood glucose monitoring, general hypoglycemic medicine and the latest guidelines, and therefore, it serves as a virtual educator delivering tailored and effective guidance for patients.
In the self‐management component, the MPA allows patients to enter daily recordings of SMBG (via Bluetooth), diet, physical activities and other diabetes data of bodyweight, blood pressure, HbA1c and more laboratory results on their mobile phones, and these data will be sent to the information center to generate into logbooks.
In the patient community component, patients can share their individual achievements and experiences with other patients in order to receive encouragement and support.
In the real‐time communication component, voice and message patterns are provided for communication after the patients are assigned to the certified clinicians by the system. The clinicians, using a professional version of the application, are available for 8 h a day/5 days a week to answer their patients' questions, and offer recommendations based on the data uploaded by patients and generated by the system. The system has a predetermined blood glucose safety value. If the blood glucose value was found to exceed the safety value, the system would automatically generate a message to the patient and notify clinicians.
Assessments
At baseline, all patients completed the diabetes management self‐efficacy questionnaire. Health and medical history were recorded. Study visits during the 24‐week period at weeks 0, 12 and 24 included assessments of HbA1c, fasting plasma glucose (FPG), 1,5‐anhydroglucitol (1,5‐AG), bodyweight, height, waist circumference and adverse events. Hypoglycemia was defined as blood glucose ≤3.9 mmol/L.
Primary study outcomes included changes of HbA1c level and the proportions of patients achieving HbA1c <7.0% in 24 weeks. Second study outcomes included changes of FPG and 1,5‐AG levels in 24 weeks.
Height and weight were measured with light clothes and without shoes. Waist circumference was measured at the level midway between the lower rib margin and the iliac crest. Hip circumference was measured at the widest part of your buttocks. Body mass index was calculated as weight (kg) divided by the square of height (m). Blood pressure was measured twice after each participant had been seated for 10 min using a mercury electric sphygmomanometer. The average was used for analysis. Venous blood samples were collected after fasting over 8 h for the measurement of FPG, triglyceride and total cholesterol, high‐density lipoprotein cholesterol, low‐density lipoprotein cholesterol and HbA1c. Extra blood samples were frozen at −80°C until assayed for 1,5‐AG.
Sample size
The study was determined based on HbA1c as the primary end‐point. Based on previous studies, with α = 0.05 and β = 0.20, a sample size of 45 patients per group was determined, considering a 10% dropout rate.
Statistical analysis
All data were analyzed using SPSS software version 19.0 (SPSS Inc., Chicago, IL, USA). Continuous data were described as the mean ± standard deviation or median (interquartile range) for specified, and categorical data were described as the number (percentages). Within‐group comparisons were carried out with paired sample Student's t‐tests (for normally distributed data) or the Mann–Whitney U‐test (for non‐normally distributed data) to evaluate the differences from baseline in each group. The analysis of variance test (for normally distributed data) or Kruskal–Wallis test (for non‐normally distributed data) were also used for comparison between the four intervention groups. Fisher's least significant difference post‐hoc test was applied for multiple comparisons where appropriate. The χ2‐test was used to analyze the differences in categorical variables. To further explore the effect of MPA or SMBG on glycemic control, we used factorial analysis of anova. The intention‐to‐treat principle was used for end‐point analyses. All reported P‐values were two sided, and P < 0.05 were considered to be statistically significant.
Results
Baseline characteristics of study participants
Of 200 patients who were screened, a total of 185 patients were enrolled and randomized to different intervention groups (Figure 1). Of these, 160 patients (86.5%) completed the study; six patients in group A, seven patients in group B, five patients in group C and seven patients in group D that terminated early with the study intervention were still encouraged to follow up the primary outcomes. The primary analysis was intention‐to‐treat and involved all patients who were randomly assigned.
Demographic and clinical characteristics are summarized in Table 1. The mean age of the patients was 52.5 years, with little difference between groups (P = 0.020). Baseline FPG averaged 8.4 mmol/L, and baseline HbA1c averaged 8.5%. There were no differences in body mass index, FPG, HbA1c, triglyceride, high‐density lipoprotein cholesterol, 1,5‐AG, proportions of HbA1c <7%, sex percentage, waist circumference, systolic blood pressure and hypoglycemic drug use between four groups (all P > 0.05), except for diastolic blood pressure and total cholesterol levels (P < 0.05).
Table 1.
Baseline characteristics of study participants of different groups
| Group A (MPA−, SMBG−) | Group B (MPA−, SMBG+) | Group C (MPA+, SMBG−) | Group D (MPA+, SMBG+) | P‐value | |
|---|---|---|---|---|---|
| n | 47 | 45 | 48 | 45 | – |
| Age (years) | 56.2 ± 8.4 | 51.4 ± 9.4* | 52.1 ± 8.9* | 50.3 ± 10.4** | 0.020 |
| M/F | 29/18 | 21/24 | 34/14 | 31/14 | 0.072 |
| BMI (kg/m2) | 25.3 ± 3.0 | 26.1 ± 4.6 | 25.4 ± 3.6 | 26.0 ± 3.9 | 0.690 |
| Waist circumference (cm) | 93.2 ± 9.1 | 91.0 ± 10.1 | 93.7 ± 12.1 | 91.5 ± 8.6 | 0.571 |
| Heart rate (b.p.m.) | 76.5 ± 7.3 | 78.0 ± 8.4 | 75.8 ± 14.9 | 79.0 ± 8.6 | 0.648 |
| SBP (mmHg) | 125.9 ± 14.7 | 124.3 ± 15.2 | 125.2 ± 22.2 | 129.9 ± 13.9 | 0.450 |
| DBP (mmHg) | 82.2 ± 8.8 | 78.2 ± 8.0 | 83.1 ± 9.5 | 85.6 ± 11.4 | 0.005 |
| FPG (mmol/L) | 8.3 ± 2.0 | 8.1 ± 2.6 | 9.3 ± 2.9 | 7.9 ± 2.2 | 0.062 |
| HbA1c (%) | 8.7 ± 1.9 | 8.6 ± 2.0 | 8.6 ± 1.8 | 8.3 ± 1.9 | 0.838 |
| HbA1c <7% (%) | 8/47 (17.0) | 12/45 (26.7) | 9/48 (18.8) | 10/45 (22.2) | 0.682 |
| TG (mmol/L) | 1.7 ± 0.8 | 1.8 ± 1.2 | 1.8 ± 0.8 | 2.2 ± 1.9 | 0.784 |
| TC (mmol/L) | 4.4 ± 0.9 | 5.0 ± 1.3 | 4.1 ± 1.1 | 3.6 ± 0.9 | 0.015 |
| HDL‐c (mmol/L) | 1.1 ± 0.3 | 1.3 ± 0.5 | 1.0 ± 0.3 | 1.1 ± 0.3 | 0.106 |
| LDL‐c (mmol/L) | 2.7 ± 0.7 | 3.3 ± 1.1 | 2.5 ± 1.2 | 2.6 ± 1.1 | 0.153 |
| 1,5‐AG† (μg/mL) | 23.0 (13.0, 31.0) | 34.0 (14.8, 68.8) | 25.0 (15.0, 45.0) | 29.0 (18.5, 68.0) | 0.416 |
| Hypoglycemic drug | |||||
| Oral drug (%) | 38.7 | 60.0 | 37.5 | 67.7 | 0.130 |
| Insulin (%) | 22.6 | 20.0 | 28.1 | 9.7 | 0.117 |
| Oral drug + insulin (%) | 38.7 | 20.0 | 34.4 | 22.6 | 0.172 |
Data are presented as the mean ± standard deviation. †Data are presented as the median (interquartile range). *Compared versus group A, P‐value <0.05. **Compared versus group A, P‐value <0.01. 1,5‐AG, 1,5‐anhydroglucitol; BMI, body mass index; DBP, diastolic blood pressure; FPG, fasting plasma glucose; HbA1c, glycated hemoglobin; HDL‐c, high‐density lipoprotein cholesterol; LDL‐c, low‐density lipoprotein cholesterol; MPA, mobile phone application; SBP, systolic blood pressure; SMBG, self‐monitoring of blood glucose; TC, total cholesterol; TG, triglyceride.
Primary study outcomes: HbA1c and the proportions of patients that achieved HbA1c <7%
The results of glycemic control over time are graphically illustrated in Table 2 and Figure 2. The HbA1c in patients of all groups decreased significantly at weeks 12 and 24 from baseline (all P < 0.01 for comparisons within groups; Figure 2a). Among the 185 patients in the analysis, the mean changes of HbA1c (%) from baseline for group A, group B, group C and group D were −1.1 ± 0.4, −1.1 ± 0.3, −1.1 ± 0.3 and −1.1 ± 0.3, respectively, at week 24, and there were no significant differences between these four groups (all P > 0.05). The proportions of patients that achieved HbA1c <7% at week 24 were 25.5% for group A, 44.4% for group B, 60.4% for group C and 62.2% for group D, respectively (Figure 2b). There were significant differences in the proportions of patients that achieved HbA1c <7% between groups, especially in group C and group D compared with group A (60.4%, 62.2% vs 25.5%, all P < 0.05) at week 24 (Table 2).
Table 2.
Changes in glycemic control at week 12 and week 24 between the four intervention groups
| Group A (MPA−, SMBG−) | Group B (MPA−, SMBG+) | Group C (MPA+, SMBG−) | Group D (MPA+, SMBG+) | |
|---|---|---|---|---|
| HbA1c (%) | ||||
| Mean level at week 12 | 7.7 ± 1.4 | 7.4 ± 1.4 | 7.4 ± 1.3 | 7.0 ± 1.1* |
| Mean level at week 24 | 7.7 ± 1.3 | 7.7 ± 1.9 | 7.3 ± 1.3 | 7.0 ± 1.2 |
| Mean change from baseline at week 12 | −1.2 ± 0.4 | −1.3 ± 0.3 | −1.1 ± 0.3 | −1.3 ± 0.4 |
| Mean change from baseline at week 24 | −1.1 ± 0.4 | −1.1 ± 0.3 | −1.1 ± 0.3 | −1.1 ± 0.3 |
| Patients achieving HbA1c <7.0% | ||||
| Week 12, n (%) | 12 (29.2) | 25 (62.5)** | 22 (51.2)* | 20 (52.6)* |
| Week 24, n (%) | 12 (25.5) | 20 (44.4) | 29 (60.4)** | 28 (62.2)** |
| FPG (mmol/L) | ||||
| Mean level at week 12 | 8.7 ± 3.0 | 7.4 ± 2.1 | 8.9 ± 3.8 | 7.1 ± 2.8 |
| Mean level at week 24 | 8.5 ± 3.6 | 8.7 ± 4.5 | 8.5 ± 3.1 | 7.2 ± 2.5 |
| Mean change from baseline at week 12 | 0.18 ± 0.68 | −0.69 ± 0.38 | 0.00 ± 0.80 | −1.36 ± 0.46 |
| Mean change from baseline at week 24 | 0.24 ± 0.79 | 0.35 ± 0.70 | −0.69 ± 0.52 | −0.65 ± 0.45 |
| 1,5‐AG (μg/mL) | ||||
| Median level at week 12† | 47.0 (19.0, 79.0) | 48.0 (19.5, 59.0) | 48.0 (22.0, 108.0) | 69.0 (34.5, 104.0) |
| Median level at week 24† | 53.0 (27.8, 62.5) | 42.0 (19, 102.6) | 42.5 (22.0, 74.8) | 71.0 (25.0, 116.0) |
| Mean change from baseline at week 12† | 4.0 (−3.0, 21.0) | 6.0 (−15.5, 11.0) | 19.0 (−0.75, 45.0) | 7.5 (−6.3, 58.3) |
| Mean change from baseline at week 24† | 15.0 (−0.5, 61.5) | 4.0 (−10.0, 13.0) | 16.5 (−7.5, 45.5) | 27.5 (−7.8, 72.3) |
Data are presented as mean ± standard deviation. †Data are presented as median (interquartile range). *Compared versus group A, P‐value <0.05. **Compared versus group A, P‐value <0.01. 1,5‐AG, 1,5‐anhydroglucitol; FPG, fasting plasma glucose; HbA1c, glycated hemoglobin; MPA, mobile phone application; SMBG, self‐monitoring of blood glucose.
Figure 2.
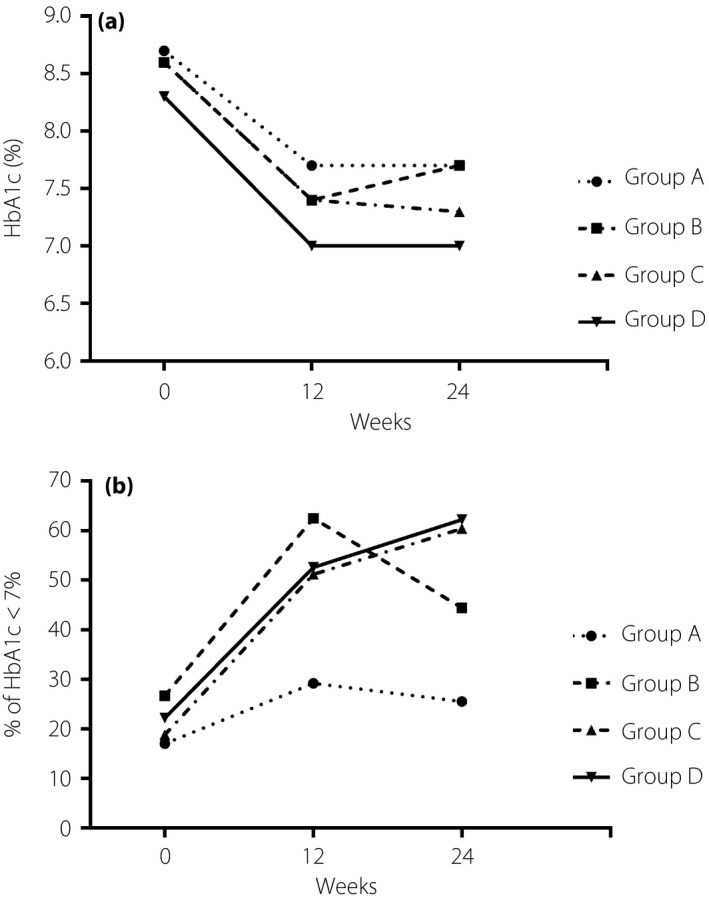
Glycemic control during 24 weeks in four groups. Group A: no mobile phone application (MPA) and no self‐monitoring of blood glucose (SMBG); Group B: SMBG only; Group C: MPA only; Group D: both MPA and SMBG were used. (a) Glycated hemoglobin (HbA1c) levels during 24 weeks in four groups. All P < 0.01 for comparisons of changes of HbA1c levels within groups at week 24. (b) The proportions of patients that achieved HbA1c <7.0% during 24 weeks in four groups. P < 0.01 for comparisons of the proportions of patients that achieved HbA1c <7.0% between groups for group C and group D at week 24.
Second study outcomes: FPG and 1,5‐AG levels
The changes of FPG levels and 1,5‐AG levels in patients of all groups during 24 weeks are shown in Table 2. The average decline of FPG levels at week 24 was larger in group C and group D than those in group A and group B, although there was no statistical significance (all P > 0.05 for comparisons between groups). The 1,5‐AG levels in patients of group A and group C were 53.0 μg/mL (interquartile range 27.8–62.5 μg/mL) and 42.5 μg/mL (interquartile range 22.0–74.8 μg/mL) at week 24, respectively, which increased significantly when compared with the levels at baseline (all P < 0.05 within groups). We also did not find differences in the median change of 1,5‐AG from baseline at week 24 between the four groups (all P > 0.05 between groups).
Impact of MPA and SMBG on glycemic control
To further explore the effect of MPA or SMBG on glycemic control, we used factorial analysis of anova (Table S1). Using HbA1c change from baseline at week 24 as the dependent variable, and MPA intervention and SMBG intervention as fixed variables, anova analysis showed that MPA intervention was the main effect of HbA1c change (F = 4.59, P = 0.034), and there was no effect on HbA1c change for SMBG intervention (P = 0.975). A similar effect can be seen in 1,5‐AG change for MPA intervention (F = 4.99, P = 0.028) and SMBG intervention (P = 0.894). There were no effects on FPG change for either MPA or SMBG intervention (all P > 0.05). There was no interaction between MPA and SMBG for HbA1c, FPG and 1,5‐AG change (all P > 0.05).
Adverse events
The major adverse event in the present study was hypoglycemia. Hypoglycemia was defined as blood glucose ≤3.9 mmol/L, and was classified as asymptomatic, documented symptomatic or severe hypoglycemia. At week 24, 10 patients in group A, three patients in group B, six patients in group C and six patients in group D reported adverse events, respectively. No severe hypoglycemia or serious adverse event occurred. None of the patients dropped out because of adverse events.
Discussion
In the present pilot study, the use of MPA, Diabetes‐Carer, was effective in improving the proportion of patients that achieved HbA1c <7% in comparison with the control group over a 24‐week study period. In addition, with factorial analysis, we conclude that MPA plays a leading role on the changes of HbA1c.
The current study did not find a significant improvement in HbA1c level in MPA groups (C and D) versus other groups (A and B). Actually, all four groups experienced a similar decrease in HbA1c level at week 24. However, the result of an improvement of HbA1c control for type 2 diabetes via mobile‐based intervention in the present study is in agreement with most previous studies, which also suggests the feasibility and availability of a mobile management platform for diabetes11, 12, 13. In current studies, the reduction of HbA1c reported by Zhou in 2016 and Gunawardena in 2018 was 1.95% and 1.36%, respectively, which were much higher than the present study11, 13. It is not surprising to see these results after considering the baseline of the present study. All patients in the four groups in the present study had moderately elevated HbA1c levels at baseline (average 8.5%), whereas one of the intervention groups (group D) had a relatively lower HbA1c (average 8.3%). The higher the baseline HbA1c level, the greater the room for control14. Another study also confirmed that a higher baseline HbA1c level is an independent predictor for blood glucose decline15. Perhaps this is one of the reasons why the present study failed to achieve a significant difference in HbA1c changes between groups.
In the present study, we use serum 1,5‐AG as a new clinical marker for the assessment of blood glycemic control. 1,5‐AG, a polyol presented in a deoxy form of glucose in the circulation, stays in a steady balance of diet intake and renal excretion regardless of HbA1c levels16. It was shown that 1,5‐AG is more sensitive to reflect postprandial blood glucose than HbA1c and glycated albumin, and to detect glucose fluctuation in the shorter term17. In some recent studies, 1,5‐AG was used as a screening tool for type 2 diabetes and gestational diabetes mellitus18, 19. Unfortunately, in the present study, in group D, which showed significant changes in the proportions of patients achieving HbA1c <7%, 1,5‐AG levels did not increase synchronously. Considering the wide range of 1,5‐AG and the confused relationship with HbA1c level, we require more studies to be carried out to understand this potential biomarker for diabetes20, 21.
The further exploration we carried out in the present study showed that MPA intervention was the main effect of HbA1c and 1,5‐AG change, and there was no interaction between MPA and SMBG. The Diabetes‐Carer application implemented in this study provides several approaches containing diabetic education, self‐management recording, patient community and real‐time communication generally. It is unclear which component contributed more to the change of HbA1c, as we did not analyze the engagement. Additionally, we cannot measure the usage of MPA by each patient. Most previous studies highlighted that self‐care activities, which contain diet and exercise patterns, and diabetes knowledge, as well as the clinical outcomes, were improved by mobile phone intervention11, 22, 23. On the contrary, one study of type 1 diabetes patients found that there was no improvement in self‐efficacy and self‐care behavior, despite a significant decrease in HbA1c15. The unexpected results were attributed to the extreme difference in engagement of each component in the MPA. Admittedly, these new interventions based on mobile phones and computers have great potential for diabetes management; however, more intelligent and automatic functions should be developed and widely used24, 25.
There were some limitations of the present study that we need to note. First, we did not quantify the changes in diabetes knowledge, diet habit, physical activities and communication rate with clinician, which were important for evaluating the effectiveness of this mobile management platform, although the glycemic control had been improved. In addition, we were unable to calculate the usage of MPA by each patient. Second, we required more evidence on special populations, such as elderly diabetes patients and type 1 diabetes patients. Third, the present study had a small sample and a short duration, after considering the chronic and long‐term characteristics of diabetes.
However, the present study found that using Diabetes‐Carer, a mobile management platform, can significantly improve the proportion of the patients achieve adequate glycemic control (HbA1c <7%). The MPA might be used as an effective and potential tool for diabetes management to improve glycemic control in China.
Disclosure
The authors declare no conflict of interest.
Supporting information
Table S1 ¦ Analysis of the effect of the mobile phone application or self‐monitoring of blood glucose on glycemic control at week 24 using two‐way anova.
Appendix S1 ¦ CONSORT 2010 checklist of information to include when reporting a randomized trial.
Acknowledgments
This study was funded by Key Specialty Construction Project of Pudong Health and Family Planning Commission of Shanghai (Grant No. PWZz2017‐12).
We were grateful to the patients, physicians and nurses in the Department of Endocrinology of Shanghai East Hospital for taking part in this trial. We were also grateful to Jie Zhou and Min Zhao from Hangzhou Kang Sheng Health Management Co., Ltd.
J Diabetes Investig 2019; 10: 1365–1371
Clinical Trial Registry Chinese Clinical Trials RegistryChiCTR‐IOR‐16008638
References
- 1. Xu Y, Wang L, He J, et al Prevalence and control of diabetes in Chinese adults. JAMA 2013; 310: 948–959. [DOI] [PubMed] [Google Scholar]
- 2. Guariguata L, Whiting DR, Hambleton I, et al Global estimates of diabetes prevalence for 2013 and projections for 2035. Diabetes Res Clin Pract 2014; 103: 137–149. [DOI] [PubMed] [Google Scholar]
- 3. Wang L, Gao P, Zhang M, et al Prevalence and ethnic pattern of diabetes and prediabetes in China in 2013. JAMA 2017; 317: 2515–2523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Muralidharan S, Ranjani H, Anjana RM, et al Mobile health technology in the prevention and management of type 2 diabetes. Indian J Endocrinol Metab 2017; 21: 334–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Coppola A, Sasso L, Bagnasco A, et al The role of patient education in the prevention and management of type 2 diabetes: an overview. Endocrine 2016; 53: 18–27. [DOI] [PubMed] [Google Scholar]
- 6. Quinn CC, Khokhar B, Weed K, et al Older adult self‐efficacy study of mobile phone diabetes management. Diabetes Technol Ther 2015; 17: 455–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Haddad NS, Istepanian R, Philip N, et al A feasibility study of mobile phone text messaging to support education and management of type 2 diabetes in Iraq. Diabetes Technol Ther 2014; 16: 454–459. [DOI] [PubMed] [Google Scholar]
- 8. Katalenich B, Shi L, Liu S, et al Evaluation of a remote monitoring system for diabetes control. Clin Ther 2015; 37: 1216–1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Quinn CC, Clough SS, Minor JM, et al WellDoc™ mobile diabetes management randomized controlled trial: change in clinical and behavioral outcomes and patient and physician satisfaction. Diabetes Technol Ther 2008; 10: 160–168. [DOI] [PubMed] [Google Scholar]
- 10. Cui M, Wu X, Mao J, et al T2DM self‐management via smartphone applications: a systematic review and meta‐analysis. PLoS ONE, 2016; 11: e0166718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Zhou W, Chen M, Yuan J, et al Welltang ‐ A smart phone‐based diabetes management application ‐ Improves blood glucose control in Chinese people with diabetes. Diabetes Res Clin Pract 2016; 116: 105–110. [DOI] [PubMed] [Google Scholar]
- 12. Kumar S, Moseson H, Uppal J, et al A diabetes mobile app with in‐app coaching from a certified diabetes educator reduces A1C for individuals with type 2 diabetes. Diabetes Educ 2018; 44: 226–236. [DOI] [PubMed] [Google Scholar]
- 13. Gunawardena KC, Jackson R, Robinett I, et al The influence of the smart glucose manager mobile application on diabetes management. J Diabetes Sci Technol 2018; 13: 75–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Quinn CC, Sareh PL, Shardell ML, et al Mobile diabetes intervention for glycemic control: impact on physician prescribing. J Diabetes Sci Technol 2014; 8: 362–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kirwan M, Vandelanotte C, Fenning A, et al Diabetes self‐management smartphone application for adults with type 1 diabetes: randomized controlled trial. J Med Internet Res 2013; 15: e235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Fujiwara T, Yoshida M, Yamada H, et al Lower 1,5‐anhydroglucitol is associated with denovo coronary artery disease in patients at high cardiovascular risk. Heart Vessels 2015; 30: 469–476. [DOI] [PubMed] [Google Scholar]
- 17. Hu X, He X, Ma X, et al A decrease in serum 1,5‐anhydroglucitol levels is associated with the presence of a first‐degree family history of diabetes in a Chinese population with normal glucose tolerance. Diabet Med 2018; 35: 131–136. [DOI] [PubMed] [Google Scholar]
- 18. Wang Y, Yuan Y, Zhang Y, et al Serum 1,5‐anhydroglucitol level as a screening tool for diabetes mellitus in a community‐based population at high risk of diabetes. Acta Diabetol 2017; 54: 425–431. [DOI] [PubMed] [Google Scholar]
- 19. Saglam B, Uysal S, Sozdinler S, et al Diagnostic value of glycemic markers HbA1c, 1,5‐anhydroglucitol and glycated albumin in evaluating gestational diabetes mellitus. Ther Adv Endocrinol Metab 2017; 8: 161–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kim MK, Jung HS, Kwak SH, et al 1,5‐Anhydro‐D‐glucitol could reflect hypoglycemia risk in patients with type 2 diabetes receiving insulin therapy. Endocrinol Metab 2016; 31: 284–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Welter M, Boritza KC, Anghebem‐Oliveira MI, et al Reference intervals for serum 1,5‐anhydroglucitol in children, adolescents, adults, and pregnant women. Clin Chim Acta 2018; 486: 54–58. [DOI] [PubMed] [Google Scholar]
- 22. Alotaibi MM, Istepanian R, Philip N. A mobile diabetes management and educational system for type‐2 diabetics in Saudi Arabia (SAED). Mhealth 2016; 2: 33–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Toma T, Athanasiou T, Harling L, et al Online social networking services in the management of patients with diabetes mellitus: systematic review and meta‐analysis of randomised controlled trials. Diabetes Res Clin Pract 2014; 106: 200–211. [DOI] [PubMed] [Google Scholar]
- 24. Nundy S, Dick JJ, Chou CH, et al Mobile phone diabetes project led to improved glycemic control and net savings for Chicago plan participants. Health Aff 2014; 33: 265–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Omar E‐G, Prem T, Nevine N, et al Mobile applications for diabetes self‐management: status and potential. J Diabetes Sci Technol 2013; 7: 247–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1 ¦ Analysis of the effect of the mobile phone application or self‐monitoring of blood glucose on glycemic control at week 24 using two‐way anova.
Appendix S1 ¦ CONSORT 2010 checklist of information to include when reporting a randomized trial.


