Figure 7. The Bd1971 linker domain and relevance to signaling.
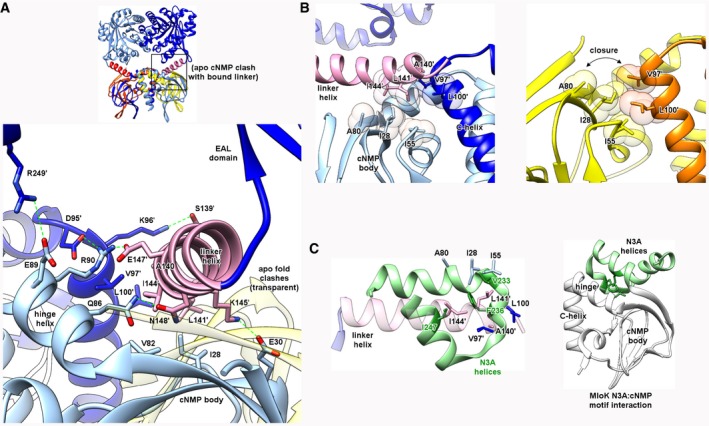
- Side chains along the full span of the linker helix contact those from both chains of the sensor dimer, forming both polar (S139, K145, E147, N148) and hydrophobic (A140, L141, I144) interfaces. The linker helix conformation in the bound state would be sterically incompatible with the position of the cNMP body in the apo state (transparent, overlaid).
- The hydrophobic A140/L141/I144 triplet forms a “wedge” that inserts between two faces formed by the cNMP body (I28, I55, A80) and C‐helix (V97′, L100′). In the apo state, this linker feature is absent, allowing direct contact of these faces (closure of cleft).
- Analogous role of N3A motif (green) from the cNMP sensor of M. loti MloK (PDB 1vp6, main fold in white). The hydrophobic patch in Bd1971 linker helix formed by A140/L141/I144 is spatially equivalent to one from MloK (V233/F236/I247) despite a difference in secondary structure between the two proteins. Similarly to Bd1971, the MloK N3A hydrophobic patch contacts the fold at a region between the hinge and cNMP body (right).
