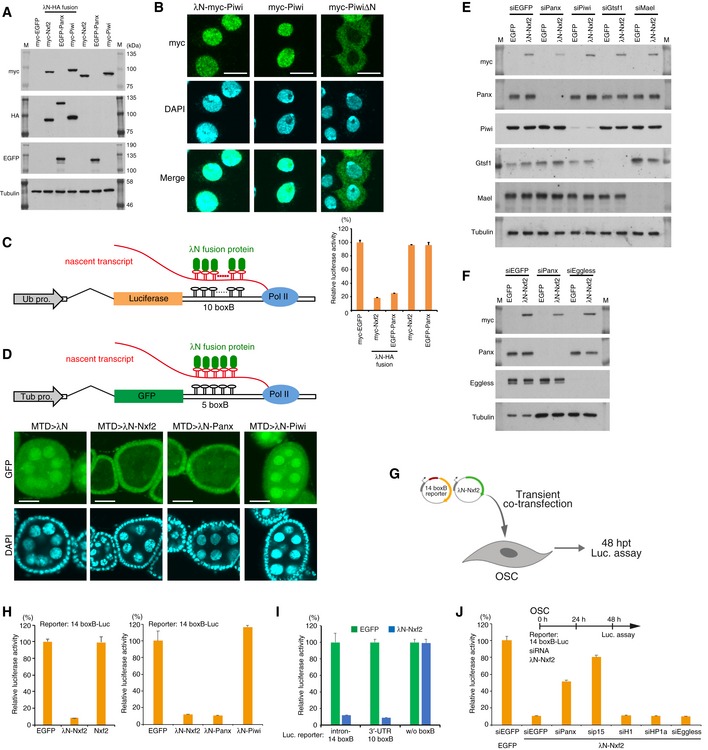
Figure EV4. Enforced tethering of Nxf2 to nascent mRNA causes co‐transcriptional silencing.
-
AWestern blotting (WB) associated with Fig 3B shows the exogenous expression of λN‐fused proteins. Nxf2 and Piwi were tagged with λN‐HA and myc, whereas Panx was tagged with λN‐HA and EGFP, since the expression level of EGFP–Panx was higher than the level of myc‐tagged Panx, which is quite unstable. M indicates protein markers.
-
BImmunofluorescence of OSCs transfected with λN‐myc‐Piwi‐, myc‐Piwi‐, or myc‐PiwiΔN‐expressing vectors, using myc antibody (Green). DAPI staining (blue) shows the location of nuclei. myc‐PiwiΔN lacking nuclear localization signal (NLS) was distributed in cytoplasm (Saito et al, 2010; Yashiro et al, 2018). λN‐myc‐Piwi localized in the nucleus similarly to myc‐Piwi, indicating that λN‐myc‐Piwi harbored piRNA in OSCs, even though it failed to induce silencing of the boxB reporter gene (Fig 3B, see also Fig EV4D and H). This may be due to only Piwi, which is guided to the target via its loaded piRNAs, potentially being able to recruit silencing machinery to the target (see also Fig EV3C and Appendix Fig S2I). Scale bars: 10 μm.
-
CSchematic of boxB‐λN tethering system in OSCs shows a genome‐integrated luciferase reporter with 10 copies of boxB sites located within the 3′ UTR of luc mRNA (left). Bar graph shows relative luciferase activity normalized by total protein amount at 48 h post‐transcription (right). Error bars indicate SD (n = 4). Tethering of Nxf2 and Panx to the 3′ UTR of luc mRNA leads to silencing in OSCs.
-
DSchematic of boxB‐λN tethering system in fly ovary shows a genome‐integrated GFP reporter with five copies of boxB sites located within the 3′ UTR of GFP mRNA. λN‐fused proteins are driven by MTD‐Gal4. Tub pro: α‐Tubulin gene promoter (top panel). Confocal images depict GFP fluorescence and DAPI signals in egg chambers expressing the indicated λN fusion proteins in the germline. Expression of λN‐Nxf2 and Panx leads to reporter silencing in germ cells, but that of λN‐Piwi does not. This is consistent with our data in Fig 3B and previous reports (Sienski et al, 2015; Yu et al, 2015). Scale bars: 20 μm.
- E, F
-
GExperimental design. According to Fig EV3B, the reporter genes do not have to be integrated into the genome, suggesting that the co‐transcriptional silencing occurs independent of the chromatin context. To examine this issue, we carried out co‐transfection of reporter plasmid (14 boxB‐Luc) and expression plasmids for λN fusion protein to OSCs, transiently. Cells were harvested at 48 hpt.
-
HEffect on luciferase activity of the proteins indicated below. Error bars indicate SD (n = 4). Even if a plasmid with 14 boxB reporter sites was transiently introduced into OSCs, λN‐Nxf2 repressed luciferase activity.
-
IλN‐Nxf2 represses luciferase activity of reporter plasmid harboring 10 boxB sites in its 3′ UTR, but not that of reporter plasmid without boxB. Error bars indicate SD (n = 4).
-
JEffects of knockdown of the indicated genes on boxB reporter activity upon λN‐Nxf2 expression. Bar graph shows luciferase activity relative to that of the sample co‐transfected with myc‐EGFP and siEGFP (control). Error bars indicate SD (n = 4). Transfection schedule of siRNA and reporter plasmids is shown at the top of the figure. Although knockdown of Panx and p15 weakened the repression by the forced tethering of Nxf2, the effects of H1‐ and HP1a‐KD on the λN‐Nxf2‐mediated silencing were negligible, which is consistent with the results from Figs 4D and EV3F.
