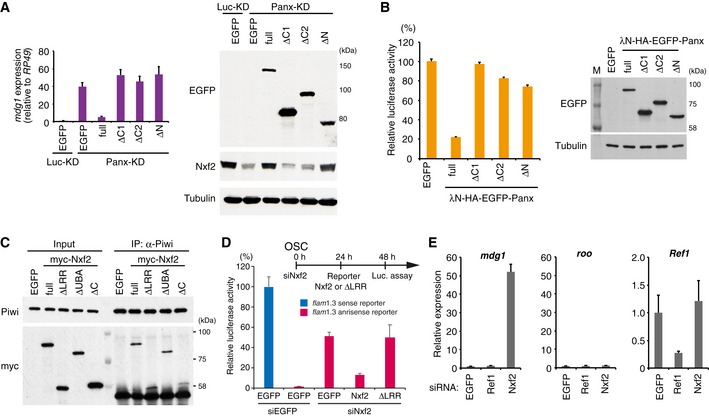
EGFP‐tagged Panx deletion constructs were expressed in Luc‐ (control) or Panx‐depleted OSCs. mdg1 expression levels were monitored by qRT–PCR. Expression values are normalized by the expression of RP49. Error bars indicate SD (n = 3) (left panel). Western blotting (WB) with an antibody against EGFP, Nxf2, and Tubulin using lysates from transfected OSCs (right panel). The deletion mutants that cannot interact with Nxf2 or Panx could not induce silencing of TE. In addition, the C‐terminal region of Panx (400–541 aa) is required for silencing of TE and stable expression of Nxf2.
Luciferase fluorescence level in OSCs expressing boxB reporter luciferase and the indicated λN fusions (Panx deletion mutants). Error bars indicate SD (n = 4) (left panel). WB of lysates from OSCs expressing boxB reporter luciferase and the indicated λN fusions using EGFP and tubulin antibody (right panel). The deletion mutants that cannot interact with Nxf2 or Panx could not induce silencing upon recruitment to reporter RNA.
Immunoprecipitation (IP) from lysate of OSCs expressing myc‐tagged Nxf2 proteins using anti‐Piwi antibody, followed by WB using anti‐myc and ‐Piwi antibodies. All mutant proteins of Nxf2 can interact with Piwi in OSCs.
Silencing of reporter gene harboring flam element (1.3 kbp, antisense) occurs in an Nxf2‐dependent manner. Transfection schedule of siRNA and plasmids is shown at the top of the figure. Exogenous expression of Nxf2 protein repressed reporter gene activity in OSCs, but that of Nxf2‐ΔLRR protein did not. Error bar indicates SD (n = 3).
RNA levels of mdg1, roo, and Ref1 were quantified by qRT–PCR upon depletion of EGFP (control), Ref1, or Nxf2. Expression levels are normalized by the expression of RP49. Error bars represent SD (n = 3). Although Ref1 knockdown significantly decreased the expression level of Ref1, mdg1 TE was unaffected.