Abstract
Purpose
For clinical implementation, a chemical exchange saturation transfer (CEST) imaging sequence must be fast, with high signal‐to‐noise ratio (SNR), 3D coverage, and produce robust contrast. However, spectrally selective CEST contrast requires dense sampling of the Z‐spectrum, which increases scan duration. This article proposes a compromise: using a 3D snapshot gradient echo (GRE) readout with optimized CEST presaturation, sampling, and postprocessing, highly resolved Z‐spectroscopy at 3T is made possible with 3D coverage at almost no extra time cost.
Methods
A 3D snapshot CEST sequence was optimized for low‐power CEST MRI at 3T. Pulsed saturation was optimized for saturation power and saturation duration. Spectral sampling and postprocessing (B0 correction, denoising) was optimized for spectrally selective Lorentzian CEST effect extraction. Reproducibility was demonstrated in 3 healthy volunteers and feasibility was shown in 1 tumor patient.
Results
Low‐power saturation was achieved by a train of 80 pulses of duration tp = 20 ms (total saturation time tsat = 3.2 seconds at 50% duty cycle) with B1 = 0.6 μT at 54 irradiation frequency offsets. With the 3D snapshot CEST sequence, a 180 × 220 × 54 mm field of view was acquired in 7 seconds per offset. Spectrally selective CEST effects at +3.5 and –3.5 ppm were quantified using multi‐Lorentzian fitting. Reproducibility was high with an intersubject coefficient of variation below 10% in CEST contrasts. Amide and nuclear overhauser effect CEST effects showed similar correlations in tumor and necrosis as show in previous ultra‐high field work.
Conclusion
A sophisticated CEST tool ready for clinical application was developed and tested for feasibility.
Keywords: APT, brain tumor, chemical exchange saturation transfer, magnetization transfer, NOE
1. INTRODUCTION
Chemical exchange saturation transfer (CEST) allows for indirect detection of diluted molecules by their saturation transfer to the abundant water pool.1, 2, 3 Many different diluted solutes were reported to be detectable with CEST such as peptides and proteins,3, 4, 5, 6 with dependency on protein conformation,7, 8, 9 creatine,10, 11 glutamate,12, 13 or even injected solutes such as iopamidol,14, 15, 16 glucose,17, 18, 19, 20 and glucose derivatives.21, 22
Regarding CEST imaging applications, there are usually competing interests of radiologists and medical physicists. Radiologists want reliable image contrasts at clinical field strengths in negligible scan time, with high signal‐to‐noise ratio (SNR) and 3D coverage. On the other hand, physicists aim to sample the Z‐spectrum with high spectral resolution in order to facilitate accurate and reliable fitting of model peaks known to exist from studies at ultra‐high field (UHF) strengths. UHF studies at 9.4 and 7T have revealed spectrally selective CEST effects related to proteins, including amide, amine, and nuclear overhauser effect (NOE) effects, as well as smaller peaks potentially related to creatine and choline phospholipids.10, 11, 23, 24 Moreover, spatial correlations between isolated amide CEST and gadolinium ring enhancement have been reported.5, 6 Changes in NOE were also shown to correlate with histology25 and be a measure for brain tumor therapy response at 7T.26 Therefore, there is clinical interest in isolation of spectrally selective CEST effects at lower field strengths.
Because of peak broadening and lower SNR at clinical field strengths, the frequency separation of different CEST effects is difficult. Many previously published applications of CEST evaluate Z‐spectrum asymmetry (MTRasym) to identify CEST contrast while removing direct water saturation effects. MTRasym approaches can be performed with a limited number of scans and are therefore relatively fast and can be performed clinically in 3D mode.27, 28 An important measure for CEST sequence efficiency is the acquisition time per irradiation frequency offset, which is currently around 30 seconds per offset for 3D CEST sequences at 3T.29 Spectrally selective CEST, on the other hand, needs more spectral samples and therefore longer scan time, which is often traded for single‐slice measurements with 4 seconds30 to 11.25 seconds31 per offset. In this work, we show that a snapshot 3D readout32 and optimized saturation scheme can be used to obtain CEST contrast with an acquisition time of 7 seconds per irradiation frequency offset. With this more‐efficient acquisition, more time can be invested to sample the Z‐spectrum finely, allowing for use of more‐advanced CEST metrics and resulting in isolated CEST effects at 3T with similar contrast to those previously observed at 9.4T.
At 3T, slow exchanging pools (e.g., NOE and amides) benefit in both spectral selectivity as well as effect strength when going to lower saturation powers. Herein, we analyzed the dependency of CEST effects as a function of saturation power and saturation time with regard to effect strength as well as spectral selectivity. We show that separation of NOE and amide signals is possible and can be robustly mapped using sophisticated postprocessing. Using the snapshot readout revealed that these NOE‐ and amide‐CEST maps can be acquired in 3D without compromising scan time. This capability enables volumetric coverage of lesions and motion correction, which is important for future clinical studies.
2. METHODS
2.1. In vivo measurements
Imaging was performed on a 3T whole‐body MRI system (MAGNETOM Prisma; Siemens Healthcare, Erlangen, Germany) on 3 healthy volunteers and 1 patient with a brain tumor with informed consent and approval by the local ethics committee. The vendor’s Head/Neck 64 coil (64 channels) was used for signal reception.
The CEST sequence consisted of a presaturation module followed by a single‐shot radiofrequency (RF) and gradient spoiled gradient‐echo readout using centric spiral reordering. The imaging parameters were field of view = 220 × 180 × 54 mm3 and matrix size 128 × 104 × 18 for 1.7 × 1.7 × 3 mm3 resolution, thus with acceleration factor Ry = 2 and elliptical scanning mode (factor 4/π more k‐space lines) a phase‐encoding matrix of 41 × 18 for a total of 738 acquired k‐space lines, TE = 2 ms, TR = 4 ms, bandwidth (BW) = 700 Hz/pixel, 18 slices, (FA) = 6°, and elongation factor E = 0.5 (rectangular spiral according to a previous work32). These settings resulted in a readout time of tRO = 738 × 0.004 seconds = 2.9 seconds.
The spectrally, but not spatially, selective CEST saturation module was optimized experimentally in a healthy volunteer by varying the pulse duration and saturation power for a train of 180° pulses. The optimal saturation period later applied for reproducibility and patient measurements consisted of a train of 80 or 100 Gaussian‐shaped RF pulses with pulse time tpulse = 20 ms, interpulse delay tdelay = 20 ms, resulting in total saturation time tsat = 3.2 to 4 seconds, and B1,mean = 0.6 µT. After the pulse train, a crusher gradient was applied to destroy spurious transversal magnetization before the next saturation block. Z‐spectrum data were obtained after saturation at 54 irradiation frequency offsets: –300 ppm for an unsaturated reference image, ±100 ppm, ±50 to ±20 ppm in steps of 10 ppm, ±10 to ±1 ppm in steps of 0.5 ppm, and –0.5 to 0.5 ppm in steps of 0.25 ppm. A similar saturation module was used previously at 7T with a single‐slice readout.5, 6, 33 Acquisition time per offset was TA = tsat + tRO = 6.1 to 6.9 seconds. For 54 irradiation frequency offsets, plus acquisition of the generalized autocalibrating partially parallel acquisition (GRAPPA) calibration kernel (32 × 18 lines) followed by 12‐second recovery time before the first CEST module, this yielded a total scan time of approximately 5.8 to 6.5 minutes for the total high‐resolution CEST‐spectrum scan. For validation of CEST contrasts found at 3T, 1 subject was additionally scanned with a similar low‐power Z‐spectrum protocol previously optimized at 9.4T.23, 34
Images were reconstructed offline using a k‐space filter and adaptive coil combination.35 Reconstructed 4D data sets were motion corrected using the AFNI 3Dvolreg function.36
2.2. Data evaluation
CEST images were generated from the Z‐value that is given by the fraction of the saturated image Ssat and the fully relaxed image S0 (Equation (1)):
| (1) |
Spectrally selective CEST effects were evaluated using Lorentzian line fitting in a 4‐step process:
2‐pool Lorentzian fitting for B0 determination
B0 correction and denoising of data
2‐pool Lorentzian fitting on B0 corrected data, generation of MTRLD
3‐pool Lorentzian fitting of MTR LD for isolated CEST contrast
In step 1, a 2‐pool model was used to describe the background signals direct water saturation and semisolid magnetization transfer (ssMT). Only irradiation frequency offsets assumed to be effected solely by the background signal were used for the fit (ssMT: ±100, ±50, ±40, ±30, ±20, –10, ±9.5, –9, ±8.5, +7.5, and +6.5 ppm; water: ±1, ±0.5, ±0.25, and 0 ppm); interleaved sampling allowed for more‐stable simultaneous fitting of ssMT and broadened CEST effects in the 3T spectrum. Other irradiation frequency offsets were excluded. The utilized 2‐pool fit model is given by the direct water saturation (w) and ssMT (Equation (2)):
| (2) |
with a constant c and the adjusted Lorentzian Lw of the water line. Lw includes a plateau to account for the pulse bandwidth at 3T defined by Equation (3):
| (3) |
where is the Lorentzian amplitude, is the Lorentzian width, and is the center frequency. Here, is the Heaviside function with and . The parameter BW accounts for the width of the plateau and is an estimation of the Fourier width of the Gaussian saturation pulse and was kept constant at . The second pool representing ssMT is a Lorentzian function defined by Equation (4):
| (4) |
The Lorentzian ssMT pool was allowed to be centered off‐resonant between 0 and –2.5 ppm. Although super‐Lorentzian line shapes were also investigated for ssMT fitting (see Supporting Information), the proposed Lorentzian line shapes were found to be sufficient and robust. Note that only symmetric super‐Lorentzian line shapes were investigated because of ambiguity in modeling and interpolation in simulated low‐power line shapes (see Supporting Information Figure S6).
In step 2, the off‐resonance of the water pool in the initial 2‐pool model was used as a surrogate B0 map, and Z‐spectra were shifted to correct for field shifts. These B0‐corrected Z‐spectra were then spectrally denoised using principal component analysis (PCA), retaining 9 of 53 components (details provided in Supporting Information Figures S7–S10).
For peak‐selective CEST evaluation, the Lorentzian difference method was used according to a previous work5 (Equation (5)):
| (5) |
Here, Zfit,ref is the 2‐pool background fit, which was repeated on the B0‐corrected and denoised Z‐spectra in step 3.
Finally, in step 4, a 3‐pool Lorentzian model was used to fit the MTRLD spectrum at offsets +10, +9, ±8, –7.5, ±7, –6.5, ±6, ±5.5, ±5, ±4.5, ±4.0, ±3.5, ±3.0, ±2.5, ±2.0, and ±1.5 ppm (those previously excluded from the background fit) to isolate the amide (+3.5 ppm), amine (+2.0 ppm), and NOE (–3.5 ppm) resonances (Equation (6)):
| (6) |
and (Equation (7))
| (7) |
All pool‐fitting parameters can be found in Supporting Information Tables S3 to S6. Quantitative maps were generated from the fitting parameter Ax for the three CEST pools and the ssMT pool. All multi‐Lorentzian fitting was performed in MATLAB (The Mathworks, Inc., Natick, MA); code is available online at www.cest-sources.org. The total postprocessing time, including B0 estimation and correction, denoising, background signal removal, and CEST pool fitting on each 4D stack, was approximately 7.5 minutes using parallel computation on a 12‐core, 2.2‐GHz computer.
Region of interest (ROI) analysis of CEST effects was performed in gray and white matter segments of a single slice in each subject. Tissue segments were approximated by thresholding the ssMT pool map. Slices for tissue analysis were chosen to ensure similar tissue distributions in each subject. Coefficient of variation across subjects (standard deviation divided by the mean) was used to determine reproducibility of fitted pool sizes.
3. RESULTS
3.1. Contrast and imaging optimization
Optimal B1 saturation power was determined by repeating measurements in a healthy subject at different B1 levels. Figure 1 shows representative B0‐corrected Z‐spectra and MTRLD spectra in gray and white matter ROIs for different B1 power levels. As B1 was increased from 0.3 μT to 0.8 μT, saturation at +3.5, +2.0, and –3.5 ppm increased, as did the broader background signal (Figure 1A,B). Removal of the direct water and ssMT contributions (Figure 1C,D) revealed increasing CEST effects with increased B1 power, which were spectrally selective at +3.5, +2.0, and –3.5 ppm. However, gray matter/white matter contrast (Figure 1E) at the +3.5‐, +2.0‐, and –3.5‐ppm resonances was optimized for B1 = 0.6 μT. The B1 = 0.6 μT power was also consistent with the 180° pulse shown previously to be most efficient.37 Figure 1F–I shows that the spectrally selective CEST contrast with B1 = 0.6 μT was maintained using a saturation train of 100, 80, and 70 Gaussian pulses at 50% duty cycle (tsat = 4, 3.2, and 2.8 seconds, respectively), improving clinical feasibility with shorter scan time.
Figure 1.
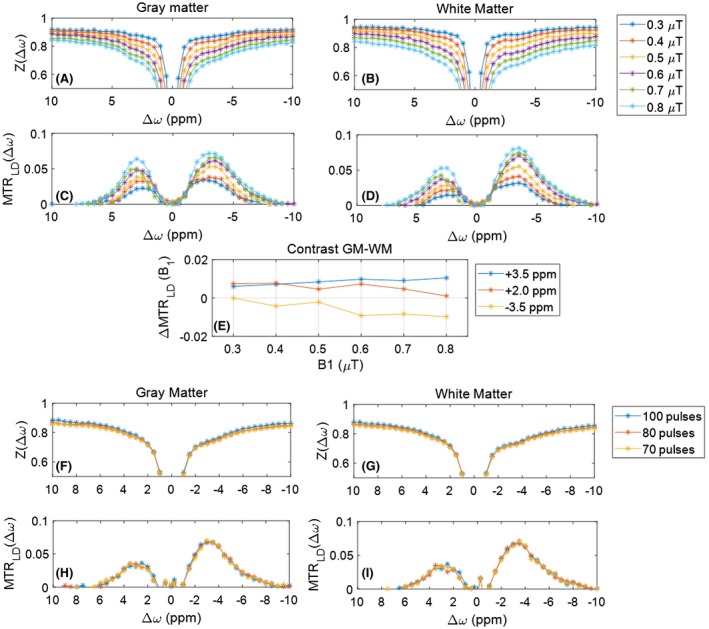
Contrast optimization. B1 dispersion of Z‐spectra (A,B) and MTRLD effects (C,D) in gray matter (GM) and white matter (WM) show that higher power with shorter pulses broadens the CEST peaks. Spectrally selective CEST effects are maximized at 0.8 μT, whereas GM‐WM contrast at +3.5, +2, and –3.5 ppm is maximized at 0.6 to 0.7 μT (E). The dependency of Z‐spectra (F,G) and CEST effects (H,I) on total saturation time is negligible. The same saturation and contrast can be sufficiently achieved with shorter saturation time, improving clinical feasibility. Z‐spectra and MTRLD spectra are in arbitrary units
3.2. CEST contrasts
Background fitting, shown in Figure 2, was achieved in a stable 2‐pool fit. The addition of the BW parameter in the direct water saturation allowed for good approximation of the width of the water peak (Figure 2B,C), and far off‐resonant points enabled stable fitting of the ssMT pool (Figure 2A,D). More information on the background fit optimization can be found in Supporting Information Figures S1–S6.
Figure 2.

Background signal fitting. (A,B) The total background signal (blue), with contributions from direct water saturation (DS; orange) and the ssMT pool (yellow), is fitted according to Equations –4. Points between ±0.5 and ±10 ppm where CEST effects are expected to occur (crosses) were excluded from the background fit. (C,D) Fitted DS and ssMT pool amplitudes in arbitrary units
The residual between the background fit (red line in Figure 2) and the measured Z‐spectra data, also referred to as the Lorentzian difference spectrum or MTRLD, revealed spectrally selective CEST effects shown in Figure 3. Lorentzian difference images at individual irradiation frequency offsets already revealed clear gray/white matter contrast at the +3.5‐ and –3.5‐ppm resonances. Whereas the MTRLD at +2.0 ppm appeared slightly increased in gray matter compared to white matter, anatomical structure was not immediately discernable and values in ROIs were not statistically significant (see Figure 3D).
Figure 3.

CEST effect fitting of Lorentzian difference spectrum (MTRLD). The Lorentzian difference signal (Equation (5)) reveals CEST contrasts at individual offsets (A–C) and in the MTRLD spectrum in gray and white matter segments in a single slice (D). Error bars represent standard deviation across gray and white matter voxels, respectively, in a single subject. MTRLD is expressed in arbitrary units
The result of fitting the MTRLD spectrum with 3 Lorentzian pools is shown in 3 volunteers in Figure 4A–C. Whole‐brain images are provided in Supporting Information Figure S12. Fitted CEST effects at +3.5 and –3.5 ppm followed the expected contrast distribution in gray and white matter. The fitted +2.0‐ppm CEST contrast was heavily influenced by direct water saturation. Additionally, residual off‐resonance effects from the background signal removal procedure obscured the contrast between gray and white matter. Figure 4D–F shows that mean MTRLD spectra in gray and white matter segments in 3 healthy subjects were in good agreement. Coefficient of variation across subjects was less than 10% for fitted +3.5‐ppm, –3.5‐ppm, and ssMT pool sizes in both gray and white matter, indicating good reproducibility of these effects. However, coefficient of variation of the fitted +2.0‐ppm resonance amplitude was 68% in gray matter and 60% in white matter.
Figure 4.

Reproducibility in 3 healthy subjects. (A–C) Isolated CEST effects obtained from 3‐pool Lorentzian fitting of the MTRLD spectrum (Equations (6) and (7)). Whole‐brain images are provided in Supporting Information Figure S12. The +2.0‐ppm signal is strongly influenced by direct water saturation and residual off‐resonance effects. (D,E) Mean MTRLD spectra in gray and white matter segments. (F) Mean and standard deviation across subjects of fitted CEST effects in gray and white matter segments of a single slice. Slices were selected to have similar gray and white matter segments for each subject. Gray matter and white matter signals are clearly distinguishable for the +3.5‐ppm, –3.5‐ppm, and ssMT pools, with coefficient of variation for each pool and each ROI printed above each bar. MTRLD spectra and fitted pool amplitudes are expressed in arbitrary units
An additional scan at 9.4T was performed for 1 subject with a similar saturation scheme.23 This allowed for direct validation of 3T spectrally selective CEST contrasts. Figure 5 shows that although 3T CEST effects were small, broad, and coalesced, the fitted contrasts at +3.5 ppm, –3.5 ppm, and ssMT were similar using the optimized 3T protocol to those found at 9.4T. Whereas UHF strength provides higher SNR, the use of spectral denoising on the highly sampled 3T data allowed for comparable anatomical detail in these contrasts. However, the slight gray/white matter contrast visible in the +2.0‐ppm resonance at 9.4T could not be replicated at 3T using the current saturation scheme and postprocessing.
Figure 5.

Comparison of spectrally selective CEST effects at 3 and 9.4T. (A,B) Z‐spectra acquired at 3T show broader, coalesced effects compared to those acquired with similar saturation parameters at 9.4T. Still, fitted CEST contrast at 3T (C) shows similar distribution as observed at 9.4T (D), confirming spectral selectivity and indicating detectable effect size at +3.5 and –3.5 ppm. UHF strengths provide much higher SNR in comparison to 3T. Denoising 3T data allows for comparable contrast. Z‐spectra and fitted pool amplitudes are expressed in arbitrary units
3.3. Tumor patient
Fitted CEST contrasts in a brain tumor patient are shown in Figure 6. Gray matter/white matter contrasts were similar in the ssMT, +3.5‐ppm, and –3.5‐ppm pools as in the healthy subjects. Similar to previous findings at UHF, the isolated 3T CEST effects showed some correlations between the amide signal and the gadolinium ring enhancement and depleted NOE signals in the tumor area and especially the necrotic region (Figure 6A).5, 23 As shown in Supporting Information S14, this information is now available in multiple slices where similar observations can be made and the full tumor can now be characterized. ROIs in the tumor core, the enhancing region, and contralateral white matter region, all of around 20 pixels in size, were defined in the coregistered contrast‐enhanced T1‐weighted image for additional analysis. In the contrast‐enhancing region the +3.5‐ppm CEST contrast was significantly elevated with (4.2 ± 0.1)% compared to contralateral white matter (2.6 ± 0.1)% (mean and standard error). NOE CEST was almost unchanged with (5.8 ± 0.2)% in the contrast‐enhancing region compared to (6.0 ± 0.1)% in the contralateral white matter. In the tumor core, the +3.5‐ppm CEST contrast was slightly elevated compared to contralateral white matter with (3.1 ± 0.1)% effect whereas the NOE CEST effect was significantly lower with (4.3 ± 0.2)%. Fitted CEST effects in all ROIs and for all contrasts are provided in Supporting Information Figure S13.
Figure 6.

Isolated CEST effects in a patient with a brain tumor for a single slice. Images from surrounding slices are provided in Supporting Information Figure S14. Postcontrast T1‐weighted images show gadolinium ring enhancement, which coincides with hyperintensity at +3.5 ppm and isointensity at –3.5 ppm, and T1 hypointensity corresponding to reduced signal in ssMT and –3.5‐ppm CEST maps. All pool sizes are expressed in arbitrary units
4. DISCUSSION
The aim of the present work was to investigate whether the spectrally selective CEST effects, which have been observed and isolated at UHF, can also be obtained at clinical field strengths with a 3D method in a clinically justifiable time. Here, we demonstrate that this goal can be achieved by utilizing a fast 3D snapshot readout and strategically selected dense sampling of the Z‐spectrum.
Because of the lower frequency separation at 3T, the CEST peak isolation is more difficult in comparison to UHF. However, a low‐power saturation scheme in association with high‐spectral‐resolution sampling of the Z‐spectrum allowed for discerning CEST effects when using a multistage Lorentzian fitting of the acquired signals. Using this approach, both amide CEST and NOE‐CEST could be isolated and showed similar contrast in healthy volunteers and a tumor patient as reported previously at higher field strengths.4, 5, 23, 32
4.1. CEST contrasts at 3T
The amide‐CEST effects reported here are difficult to compare to the well‐established amide‐proton transfer‐weighted (APTw) scans performed at 3T, given that APTw experiments use high‐power pulses and thus have a higher labeling and stronger weighting of faster exchanging protons. Despite that, the principle result of increased amide CEST effect in the tumor area30 were also observed by the present spectrally selective low‐power approach. Still, the tumor contrast in the isolated amide CEST signal was not as strong as in APTw imaging, and gray matter and tumor regions were also similar in intensity, which is in agreement with previous observations at UHF.5 Thus, it remains to be investigated whether the isolated amide CEST gives a clinical benefit over APTw MRI at 3T.
The isolated NOE‐CEST contrast, on the other hand, appeared to be aided by using the low‐power approach compared to previous work at 3T. Heo et al30 used a higher power where the NOE is suppressed and an effect strength of only around 2% in white matter is reported, compared to our average effect strength of 6%. Shen et al38 used similar saturation power to the current study, but longer saturation time and single‐slice measurements. The NOE images obtained in our study had better quality in terms of SNR and resolution, as well as stronger NOE effect isolated by Lorentzian fitting.
A similar low‐power, high‐spectral‐resolution protocol was recently applied to brain tumors by Desmond et al.31 and Mehrabian et al.39, 40 They reported an amide‐CEST effect of 3.4% and NOE‐CEST effect of 7.3% in white matter, comparable to our findings. In tumors, they found both effects to be decreased. Notably, their approaches were single‐slice measurements with at least 4 averages resulting in scan times reaching up to 12 minutes. Compared to single‐slice methods, the 3D snapshot CEST approach used in this study improves SNR and coverage. Additionally, denoising using PCA reduced the number of required averages (see Supporting Information Figure S10), thereby making volumetric coverage available in clinically feasible examination times.
The snapshot CEST readout used in this work has been adapted from its original implementation at 9.4T.23, 32 The readout was accelerated and centric spiral reordered, but still Cartesian, facilitating reconstruction at the scanner. The current implementation is a proof of concept demonstrating that a fast 3D readout can be used to isolate even small effect sizes at clinical field strengths. The signal of the snapshot gradient echo readout will decay with the Look‐Locker relaxation time influenced both by T1 relaxation and by excitation. With the current readout length of 738 k‐space lines in 2.9 seconds, the actual resolution of the CEST images was reduced by a factor of 1.5 because of blurring of the point spread function. The readout used in this study was chosen as a trade‐off between volume coverage and actual resolution; details about this trade‐off are described in previous work.32 Blurring can be reduced with a shorter readout at the cost of reduced resolution or volume coverage.
To adjust for lower SNR available at 3T, the current implementation used larger voxels, reduced acceleration, and a higher FA. It is possible to gain additional signal by reducing the readout BW or increasing the slice thickness. Note, however, that denoising of Z‐spectra by methods as simple as PCA could improve the robustness of modelling of small‐amplitude CEST signals at 3T. After denoising, adequate signals from amides and NOEs emerged with comparable contrast to that obtained at UHF. Moreover, these signals were robustly altered in the presence of pathology.
The present snapshot CEST approach measured approximately 7 seconds per irradiation frequency offset, whereas 30 seconds per irradiation frequency offset were necessary in recent 3D APTw imaging approaches.29 Note also that other saturation modules, such as APT‐weighted, glucose‐enhanced, or glutamate presaturation, are compatible with the snapshot 3D gradient echo readout.
4.2. Saturation pulse duration and power level
In this study, we found that saturation times of 2.8 to 4 seconds, reflecting Tsat of 2.15·T1 to 3·T1 for gray matter at 3T, yielded similar CEST effect sizes. This observation agreed with pulsed CEST findings at 9.4T,23 which showed that spectrally selective CEST effect sizes plateau at Tsat > 1.7·T1, with reduced effects with less than 3 seconds of saturation.
The B1 dispersion experiment in this study indicated that saturation pulses with powers in the range of 0.6 to 0.8 µT seem to be optimal for isolating CEST effects at +3.5 and –3.5 ppm. Higher B1 powers may have more exchange weighting, but at the cost of spectral selectivity; this phenomenon was also observed in the human brain at 9.4T.23 The B1 inhomogeneity at 3T caused only 9% reduced CEST effect at the +3.5‐ppm resonance and was only observed in the frontal brain regions (see Supporting Information Figure S12). Although no B1 correction was performed for the 3T data in this study, it can be implemented at the cost of scan time because of the need for repeated measurements.33
Here, we used short Gaussian shaped pulses with 180° effective FA, which was reported to yield more CEST contrast.37 However, short saturation pulses may not be optimal for isolation of the +2.0‐ppm signal. Maps of isolated CEST contrasts shown in Figure 4 indicate that the fitted +2.0‐ppm resonance was influenced by residual B0 inhomogeneity. Removing the +2.0‐ppm signal from the MTRLD fit resulted in these spillover effects appearing in the +3.5‐ppm fitted contrast (see Supporting Information Figure S11). With a 3‐pool model, the gray matter/white matter contrast in the +3.5‐ppm CEST effect was more stable, but a trade‐off was observed between fitting of the +3.5‐ and +2.0‐ppm resonances, indicating that these effects overlapped using the current saturation scheme. The approach of Desmond et al.31 used longer pulse durations and more‐dense frequency sampling between ±0.5 and ±6 ppm, which allowed for better isolated amine contrast at +2.0 ppm than that obtained in the present study; such an approach could easily be applied with the 3D snapshot CEST readout. However, longer pulses might also lead to lower labeling as a narrower BW of CEST frequencies is labeled.41, 42 An adiabatic off‐resonant spin lock pulse43 with low direct saturation may also be of interest at lower field strengths for investigating resonances near water.
4.3. Fit model
In this study, we used a 2‐stage fitting approach for improved separation of CEST effects from direct water saturation and broad semisolid MT effects. This approach, which previously yielded spectrally selective CEST effects at 9.4T in mice24 and humans,23 also resulted in stronger contrast at 3T compared to 3‐point fitting.38 Measurement of far off‐resonant frequency offsets beyond ±6 ppm in particular facilitated the estimation of the ssMT contribution. Direct multi‐Lorentzian fitting has also been applied in 3T low‐power experiments,31, 40 where the ssMT contribution was modeled as a constant offset in the Z‐spectrum between ±6 ppm. The approach used in the present study allowed for asymmetric ssMT contributions, which is important especially for the separation of ssMT and NOE‐CEST contributions.
4.4. Outlook and clinical translation
It has been shown that the downfield amide proton signal is sensitive to pH, whereas the upfield NOE signal is pH‐insensitive44 and may be related to protein content25 and protein denaturation7 in brain tumors. Providing 3D coverage in less than 7 minutes of additional scan time, the snapshot CEST approach presented here can facilitate clinical translation of endogenous protein CEST contrast at 3T. With the addition of T1 mapping, relaxation compensation5 and downfield NOE suppression6 analysis can be readily achieved.
5. CONCLUSION
We have demonstrated feasibility of spectrally selective CEST imaging of NOE and amide effects at clinical field strength using a fast 3D snapshot CEST sequence, enabling full Z‐spectrum sampling with volumetric coverage in a clinical time frame. Using an optimized saturation scheme with low B1 power and high spectral resolution, combined with volumetric coverage and denoising, this approach allows for robust postprocessing and reproducible isolated amide‐ and NOE‐CEST contrasts in vivo. These methods provide similar gray matter/white matter contrast at 3T as can be obtained at 9.4T in healthy volunteers as well as in a tumor patient. Resonances closer to water visible at UHF strengths could not yet be robustly isolated at 3T using the current low‐power approach.
Supporting information
Figure S1 Simulated Z‐spectra in gray matter and white matter under low‐power pulsed and continuous wave (CW) saturation
Figure S2 Residual of 4 fit models to simulated continuous wave saturation at equivalent power to Supporting Information Figure S1, zoomed to show points near 0 ppm (rows 1–4) and far off‐resonance (rows 5–8)
Figure S3 Residual of four 2‐pool fit models to simulated pulsed saturation schemes, zoomed to show points near 0 ppm (rows 1–4) and far off‐resonance (rows 5–8). Total saturation time is kept constant at 4 seconds, and pulse amplitude and duration are varied to achieve approximately 180° rotation
Figure S4 In vivo MTRLD using four 2‐pool models to fit the background (DS + MT) of Z‐spectra measured using pulsed saturation schemes, zoomed to show points near 0 ppm (rows 1–4) and far off‐resonance (rows 5–8)
Figure S5 In vivo MTRLD using 2‐pool Lorentzian and super‐Lorentzian with BW model to fit the background (water + ssMT) of Z‐spectra measured using pulsed saturation (100 Gaussian‐shaped pulses, 0.6 μT, tp = 20 ms, 50 % duty cycle), zoomed to show points near 0 ppm (rows 1–4) and far off‐resonance (rows 5–8)
Figure S6 Simulated 2‐pool Z‐spectra of white matter for 0.6‐µT continuous wave saturation using the Henkelman model with a centered super‐Lorentzian MT line shape (solid black line) and shifted super‐Lorentzian MT lineshape (red dashed line). In addition to the background, a sharp peak at the MT resonance shift of –2.34 ppm is visible in the shifted line shape. This peak can be removed by interpolation of the MT line shape within an interval of ±1 ppm around the MT resonance frequency (blue solid line), but the resulting line shape depends on the arbitrarily chosen interpolation interval (blue, pink, and yellow dashed lines)
Figure S7 PCA of Z‐spectra. (a) First 4 principle components exhibiting spectral features near 0 ppm. (b) All principle components, exhibiting broad CEST spectral features between ±5 ppm (components 5–20) and noise‐like features (components 20–53)
Figure S8 Projection of data of a single slice from a healthy subject onto principle components. The first few components contain anatomical and experimentally relevant information, whereas the remaining components contribute noise
Figure S9 Eigenvalues of principle components of measured Z‐spectra in 5 healthy volunteers. The stars denote the optimal number of components based on Malinowski’s empirical indicator function
Figure S10 Effect of denoising of Z‐spectra on CEST contrast when retaining 42, 36, 27, 14, 11, 9, and 6 of 53 components compared to averaging of repeated measurements. MTRLD is expressed in arbitrary units
Figure S11 Including the +2.0‐ppm pool in the MTRLD analysis improves the stability of the fit at +3.5 ppm. Using a 2‐pool fit, the +3.5‐ppm fitted resonance is influenced by direct water saturation and residual B0 errors
Figure S12 Whole‐brain fitted CEST contrasts from which single slice images in Figure 4a to 4c were extracted
Figure S13 Mean and standard deviation of fitted Lorentzian pool sizes in single‐slice ROIs in the brain tumor patient
Figure S14 Fitted background (water, DS, and ssMT) and CEST contrasts in several slices covering the tumor. Gadolinium contrast‐enhanced images are provided for reference
Table S1 Pulsed and continuous wave power equivalent (CWPE) protocols used in Bloch simulation Abbreviations: tp, pulse duration; DC, duty cycle
Table S2 Gray matter and white matter parameters employed in Bloch simulation Values are taken from a previous work.1 Δω, frequency offset; ppm, parts per million; f, pool fraction; k, exchange rate
Table S3 Boundary and initial conditions of the 2‐pool Lorentzian model used for background fitting
Table S4 Boundary and initial conditions of the 2‐pool super‐Lorentzian model used for background fitting Pool A refers to water, pool C refers to the macromolecular pool
Table S5 Boundary and initial conditions of the 3‐pool Lorentzian model used for CEST pool fitting of MTRLD,net
Table S6 Frequency offsets used in multistage fitting
ACKNOWLEDGMENTS
The financial support of the Max Planck Society, German Research Foundation (DFG, grant ZA 814/2‐1, support to M.Z., K.H., and M.S.), and European Union’s Horizon 2020 research and innovation programme (Grant Agreement No. 667510, support to A.D., M.Z.) is gratefully acknowledged.
Deshmane A, Zaiss M, Lindig T, et al. 3D gradient echo snapshot CEST MRI with low power saturation for human studies at 3T. Magn Reson Med. 2019;81:2412–2423. 10.1002/mrm.27569
Funding Information
Max Planck Society, German Research Foundation (DFG, grant ZA 814/2‐1, support to K.H., and M.S.), and European Union’s Horizon 2020 research and innovation programme (Grant Agreement No. 667510, support to A.D., M.Z.)
REFERENCES
- 1. Forsen S, Hoffman RA. Study of moderately rapid chemical exchange reactions by means of nuclear magnetic double resonance. J Chem Phys. 1963;39:2892–2901. [Google Scholar]
- 2. Ward K, Aletras A, Balaban R. A new class of contrast agents for MRI based on proton chemical exchange dependent saturation transfer (CEST). J Magn Reson. 2000;143:79–87. [DOI] [PubMed] [Google Scholar]
- 3. Zhou J, Payen JF, Wilson DA, Traystman RJ, van Zijl P. Using the amide proton signals of intracellular proteins and peptides to detect pH effects in MRI. Nat Med. 2003;9:1085–1090. [DOI] [PubMed] [Google Scholar]
- 4. Jones CK, Huang A, Xu J, et al. Nuclear Overhauser enhancement (NOE) imaging in the human brain at 7T. NeuroImage. 2013;77:114–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Zaiss M, Windschuh J, Paech D, et al. Relaxation‐compensated CEST‐MRI of the human brain at 7T: unbiased insight into NOE and amide signal changes in human glioblastoma. NeuroImage. 2015;112:180–188. [DOI] [PubMed] [Google Scholar]
- 6. Zaiss M, Windschuh J, Goerke S, et al. Downfield‐NOE‐suppressed amide‐CEST‐MRI at 7 Tesla provides a unique contrast in human glioblastoma. Magn Reson Med. 2017;77:196–208. [DOI] [PubMed] [Google Scholar]
- 7. Zaiss M, Kunz P, Goerke S, Radbruch A, Bachert P. MR imaging of protein folding in vitro employing Nuclear‐Overhauser‐mediated saturation transfer. NMR Biomed. 2013;26:1815–1822. [DOI] [PubMed] [Google Scholar]
- 8. Goerke S, Zaiss M, Kunz P, et al. Signature of protein unfolding in chemical exchange saturation transfer imaging. NMR Biomed. 2015;28:906–913. [DOI] [PubMed] [Google Scholar]
- 9. Goerke S, Milde KS, Bukowiecki R, et al. Aggregation‐induced changes in the chemical exchange saturation transfer (CEST) signals of proteins. NMR Biomed. 2017;30:e3665. [DOI] [PubMed] [Google Scholar]
- 10. Kogan F, Haris M, Singh A, et al. Method for high‐resolution imaging of creatine in vivo using chemical exchange saturation transfer. Magn Reson Med. 2014;71:164–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Rerich E, Zaiss M, Korzowski A, Ladd ME, Bachert P. Relaxation‐compensated CEST‐MRI at 7 T for mapping of creatine content and pH—preliminary application in human muscle tissue in vivo. NMR Biomed. 2015;28:1402–1412. [DOI] [PubMed] [Google Scholar]
- 12. Cai K, Haris M, Singh A, et al. Magnetic resonance imaging of glutamate. Nat Med. 2012;18:302–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Haris M, Nath K, Cai K, et al. Imaging of glutamate neurotransmitter alterations in Alzheimer’s disease. NMR Biomed. 2013;26:386–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Aime S, Calabi L, Biondi L, et al. Iopamidol: Exploring the potential use of a well‐established x‐ray contrast agent for MRI. Magn Reson Med. 2005;53:830–834. [DOI] [PubMed] [Google Scholar]
- 15. Longo DL, Busato A, Lanzardo S, Antico F, Aime S. Imaging the pH evolution of an acute kidney injury model by means of iopamidol, a MRI‐CEST pH‐responsive contrast agent. Magn Reson Med. 2013;70:859–864. [DOI] [PubMed] [Google Scholar]
- 16. Jones KM, Randtke EA, Howison CM, et al. Measuring extracellular pH in a lung fibrosis model with acidoCEST MRI. Mol Imaging Biol. 2015;17:177–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Chan KW, McMahon MT, Kato Y, et al. Natural D‐glucose as a biodegradable MRI contrast agent for detecting cancer. Magn Reson Med. 2012;68:1764–1773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Walker‐Samuel S, Ramasawmy R, Torrealdea F, et al. In vivo imaging of glucose uptake and metabolism in tumors. Nat Med. 2013;19:1067–1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Xu X, Yadav NN, Knutsson L, et al. Dynamic glucose‐enhanced (DGE) MRI: translation to human scanning and first results in glioma patients. Tomography. 2015;1:105–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Schuenke P, Koehler C, Korzowski A, et al. Adiabatically prepared spin‐lock approach for T1ρ‐based dynamic glucose enhanced MRI at ultrahigh fields. Magn Reson Med. 2017;78:215–225. [DOI] [PubMed] [Google Scholar]
- 21. Nasrallah FA, Pagès G, Kuchel PW, Golay X, Chuang KH. Imaging brain deoxyglucose uptake and metabolism by glucoCEST MRI. J Cereb Blood Flow Metab. 2013;33:1270–1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Rivlin M, Tsarfaty I, Navon G. Functional molecular imaging of tumors by chemical exchange saturation transfer MRI of 3‐O‐Methyl‐D‐glucose. Magn Reson Med. 2014;72:1375–1380. [DOI] [PubMed] [Google Scholar]
- 23. Zaiss M, Schuppert M, Deshmane A, et al. Chemical exchange saturation transfer MRI contrast in the human brain at 9.4 T. NeuroImage. 2018;179:144–155. [DOI] [PubMed] [Google Scholar]
- 24. Cai K, Singh A, Poptani H, et al. CEST signal at 2 ppm (CEST@2 ppm) from Z ‐spectral fitting correlates with creatine distribution in brain tumor. NMR Biomed. 2014;28:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Paech D, Burth S, Windschuh J, et al. Nuclear overhauser enhancement imaging of glioblastoma at 7 Tesla: region specific correlation with apparent diffusion coefficient and histology. PLoS ONE. 2015;10:1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Meissner JE, Regnery S, Korozowski A, et al. NOE‐mediated CEST imaging of glioma at 7 Tesla aids early response evaluation of patients undergoing radio‐chemotherapy In: Proceedings of the Joint Annual Meeting ISMRM‐ESMRMB, Paris, France, 2018. p. 408. [Google Scholar]
- 27. Zhu H, Jones CK, van Zijl PC, Barker PB, Zhou J. Fast 3D chemical exchange saturation transfer (CEST) imaging of the human brain. Magn Reson Med. 2010;64:638–644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Togao O, Yoshiura T, Keupp J, et al. Amide proton transfer imaging of adult diffuse gliomas: correlation with histopathological grades. Neuro Oncol. 2014;16:441–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Togao O, Keupp J, Hiwatashi A, et al. Amide proton transfer imaging of brain tumors using a self‐corrected 3D fast spin‐echo dixon method: comparison with separate B0 correction. Magn Reson Med. 2017;77:2272–2279. [DOI] [PubMed] [Google Scholar]
- 30. Heo HY, Zhang Y, Jiang S, Lee DH, Zhou J. Quantitative assessment of amide proton transfer (APT) and nuclear overhauser enhancement (NOE) imaging with extrapolated semisolid magnetization transfer reference (EMR) signals: II. Comparison of three EMR models and application to human brain glioma at. Magn Reson Med. 2016;75:1630–1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Desmond KL, Mehrabian H, Chavez S, et al. Chemical exchange saturation transfer for predicting response to stereotactic radiosurgery in human brain metastasis. Magn Reson Med. 2017;78:1110–1120. [DOI] [PubMed] [Google Scholar]
- 32. Zaiss M, Ehses P, Scheffler K. Snapshot‐CEST: optimizing spiral‐centric‐reordered gradient echo acquisition for fast and robust 3D CEST MRI at 9.4 T. NMR Biomed. 2018;31:e3879. [DOI] [PubMed] [Google Scholar]
- 33. Windschuh J, Zaiss M, Meissner JE, et al. Correction of B1‐inhomogeneities for relaxation‐compensated CEST imaging at 7T. NMR Biomed. 2015;28:529–537. [DOI] [PubMed] [Google Scholar]
- 34. Griswold MA, Jakob PM, Heidemann RM, et al. Generalized autocalibrating partially parallel acquisitions (GRAPPA). Magn Reson Med. 2002;47:1202–1210. 10.1002/mrm.10171 [DOI] [PubMed] [Google Scholar]
- 35. Walsh D, Gmitro A, Marcellin M. Adaptive reconstruction of phased array MR imagery. Magn Reson Med. 2000;43:682–690. [DOI] [PubMed] [Google Scholar]
- 36. Cox RW, Hyde JS. Software tools for analysis and visualization of fMRI data. NMR Biomed. 1997;10:171–178. [DOI] [PubMed] [Google Scholar]
- 37. Zu Z, Li K, Janve VA, Does MD, Gochberg DF. Optimizing pulsed‐chemical exchange saturation transfer imaging sequences. Magn Reson Med. 2011;66:1100–1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Shen Y, Xiao G, Shen Z, et al. Imaging of nuclear Overhauser enhancement at 7 and 3 T. NMR Biomed. 2017;30:e3735. [DOI] [PubMed] [Google Scholar]
- 39. Mehrabian H, Lam WW, Myrehaug S, Sahgal A, Stanisz GJ. Glioblastoma (GBM) effects on quantitative MRI of contralateral normal appearing white matter. J Neurooncol. 2018;139:97–106. [DOI] [PubMed] [Google Scholar]
- 40. Mehrabian H, Myrehaug S, Soliman H, Sahgal A, Stanisz GJ. Evaluation of glioblastoma response to therapy with chemical exchange saturation transfer. Int J Radiat Oncol. 2018;101:713–723. [DOI] [PubMed] [Google Scholar]
- 41. van Zijl P, Lam WW, Xu J. Knutsson L, Stanisz GJ. Magnetization transfer contrast and chemical exchange saturation transfer MRI. Features and analysis of the field‐dependent saturation spectrum. NeuroImage. 2018;168:222–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Goerke S, Breitling J, Zaiss M, et al. Dual‐frequency irradiation CEST‐MRI of endogenous bulk mobile proteins. NMR Biomed. 2018;31:e3920. [DOI] [PubMed] [Google Scholar]
- 43. Herz K, Gandhi C, Schuppert M, Deshmane A, Scheffler K, Zaiss M. CEST imaging at 9.4 T using adjusted adiabatic spin‐lock pulses for on‐ and off‐resonant T1⍴‐dominated Z‐spectrum acquisition. Magn Reson Med. 2018. Sep 8. doi: 10.1002/mrm.27380. [DOI] [PubMed] [Google Scholar]
- 44. Jin T, Wang P, Zong X, Kim SG. MR imaging of the amide‐proton transfer effect and the pH‐insensitive nuclear overhauser effect at 9.4 T. Magn Reson Med. 2013;69:760–770. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1 Simulated Z‐spectra in gray matter and white matter under low‐power pulsed and continuous wave (CW) saturation
Figure S2 Residual of 4 fit models to simulated continuous wave saturation at equivalent power to Supporting Information Figure S1, zoomed to show points near 0 ppm (rows 1–4) and far off‐resonance (rows 5–8)
Figure S3 Residual of four 2‐pool fit models to simulated pulsed saturation schemes, zoomed to show points near 0 ppm (rows 1–4) and far off‐resonance (rows 5–8). Total saturation time is kept constant at 4 seconds, and pulse amplitude and duration are varied to achieve approximately 180° rotation
Figure S4 In vivo MTRLD using four 2‐pool models to fit the background (DS + MT) of Z‐spectra measured using pulsed saturation schemes, zoomed to show points near 0 ppm (rows 1–4) and far off‐resonance (rows 5–8)
Figure S5 In vivo MTRLD using 2‐pool Lorentzian and super‐Lorentzian with BW model to fit the background (water + ssMT) of Z‐spectra measured using pulsed saturation (100 Gaussian‐shaped pulses, 0.6 μT, tp = 20 ms, 50 % duty cycle), zoomed to show points near 0 ppm (rows 1–4) and far off‐resonance (rows 5–8)
Figure S6 Simulated 2‐pool Z‐spectra of white matter for 0.6‐µT continuous wave saturation using the Henkelman model with a centered super‐Lorentzian MT line shape (solid black line) and shifted super‐Lorentzian MT lineshape (red dashed line). In addition to the background, a sharp peak at the MT resonance shift of –2.34 ppm is visible in the shifted line shape. This peak can be removed by interpolation of the MT line shape within an interval of ±1 ppm around the MT resonance frequency (blue solid line), but the resulting line shape depends on the arbitrarily chosen interpolation interval (blue, pink, and yellow dashed lines)
Figure S7 PCA of Z‐spectra. (a) First 4 principle components exhibiting spectral features near 0 ppm. (b) All principle components, exhibiting broad CEST spectral features between ±5 ppm (components 5–20) and noise‐like features (components 20–53)
Figure S8 Projection of data of a single slice from a healthy subject onto principle components. The first few components contain anatomical and experimentally relevant information, whereas the remaining components contribute noise
Figure S9 Eigenvalues of principle components of measured Z‐spectra in 5 healthy volunteers. The stars denote the optimal number of components based on Malinowski’s empirical indicator function
Figure S10 Effect of denoising of Z‐spectra on CEST contrast when retaining 42, 36, 27, 14, 11, 9, and 6 of 53 components compared to averaging of repeated measurements. MTRLD is expressed in arbitrary units
Figure S11 Including the +2.0‐ppm pool in the MTRLD analysis improves the stability of the fit at +3.5 ppm. Using a 2‐pool fit, the +3.5‐ppm fitted resonance is influenced by direct water saturation and residual B0 errors
Figure S12 Whole‐brain fitted CEST contrasts from which single slice images in Figure 4a to 4c were extracted
Figure S13 Mean and standard deviation of fitted Lorentzian pool sizes in single‐slice ROIs in the brain tumor patient
Figure S14 Fitted background (water, DS, and ssMT) and CEST contrasts in several slices covering the tumor. Gadolinium contrast‐enhanced images are provided for reference
Table S1 Pulsed and continuous wave power equivalent (CWPE) protocols used in Bloch simulation Abbreviations: tp, pulse duration; DC, duty cycle
Table S2 Gray matter and white matter parameters employed in Bloch simulation Values are taken from a previous work.1 Δω, frequency offset; ppm, parts per million; f, pool fraction; k, exchange rate
Table S3 Boundary and initial conditions of the 2‐pool Lorentzian model used for background fitting
Table S4 Boundary and initial conditions of the 2‐pool super‐Lorentzian model used for background fitting Pool A refers to water, pool C refers to the macromolecular pool
Table S5 Boundary and initial conditions of the 3‐pool Lorentzian model used for CEST pool fitting of MTRLD,net
Table S6 Frequency offsets used in multistage fitting


