Abstract
Introduction
Patients with COPD often show increased systemic inflammation which is associated with lower functional status, greater exacerbation risk, and worse clinical outcomes. Syndecans (SDCs), a family of transmembrane heparan sulfate proteoglycans (HSPGs), have been found to involve in inflammatory processes in many chronic inflammatory diseases. The aim of this preliminary clinical study was to investigate the possible association between two SDCs, SDC-1 and SDC-4, with lung function, systemic inflammation, and risk of exacerbations in COPD patients.
Method
Serum SDC-1 and SDC-4 levels were measured in 101 COPD patients and 57 health controls. Correlations between SDCs and other parameters were analyzed using Spearsman’s rho. Receiver operating curve (ROC) analysis was used to evaluate the threshold value in differentiating disease status.
Results
Although both serum SDC-1 and SDC-4 showed a downward trend in COPD patients, only SDC-1 levels were correlated positively with the ratio of FEV1/FVC and parameters of small airway obstruction. Besides, SDC-1 but not SDC-4, was negatively correlated with C-reactive protein (CRP) in COPD patients and downregulated in frequent exacerbators (FEs) of COPD. Using a cutoff value of 2.08 ng/mL, the sensitivity and specificity of SDC-1 to differentiate FE were 44% and 93.4%, respectively.
Conclusion
In conclusion, circulating SDC-1 may be a novel inflammatory biomarker associated with lung function and systemic inflammation in patients with COPD, which could also be useful to identify the risk of COPD exacerbation. Further studies should be performed to clarify the influences of SDC-1 on the pathogenesis and outcomes of COPD.
Keywords: syndecan, chronic obstructive pulmonary disease, systemic inflammation, exacerbation, biomarker
Introduction
COPD remains a common health and social problem with high morbidity and mortality rates.1 The course of the disease will also be progressive, increasing frequency and severity of acute exacerbations (AECOPD) that are associated with a substantial increase in all-cause mortality. Besides, patients with COPD often show increased systemic inflammation apart from local inflammation in the lungs, leading to lower functional status, greater exacerbation risk, and poorer clinical outcomes.2–4 Importantly, the inflammatory responses in COPD can be self-perpetuating even in the absence of ongoing stimuli,5 which may be attributed to an amplified, inflammatory cascade mechanism involving the activation and release of several inflammatory cells and mediators. Increasing studies have shown that overexpression of proinflammatory molecules found in both lung and peripheral blood has been correlated with airway obstruction and its severity.6,7 Therefore, the identification of novel inflammatory biomarkers can not only be clinically useful for COPD stratification, but may also help to monitor the progression and manage the patients of COPD.
In recent years, increasing evidence have indicated that syndecans (SDCs), a family of transmembrane heparan sulfate proteoglycans (HSPGs), played an important role during inflammatory processes by interacting with a variety of ligands, including cytokines, chemokines, growth factors, and growth factor receptors, to maintain an inflammatory microenvironment.8 Moreover, cleavage and shedding of SDCs can mediate negative regulation of chemokine and growth factor signaling pathways and ligand sequestration.9 There are four mammalian SDCs, SDC-1 thorough 4, among which SDC-1 is the major cell surface HSPG of epithelial cells, including the airway epithelium. Previous data indicate that shedding of SDC-1 negatively regulates both infectious and noninfectious lung inflammation by modulating key inflammatory mediators.10,11 Meanwhile, circulating SDC-1 levels may mirror vascular endothelial damage and inflammation in acute systemic vasculitis.12 SDC-2 is primarily found in cells of mesenchymal origin, and SDC-3 is mainly expressed by neuronal tissue. SDC-4, on the other hand, is expressed by most cell types and has been found to be involved in tumor metastasis, angiogenesis, and inflammation. Systemic administration of SDC-4 caused a substantial increase in the number of bronchiolar progenitors with concomitant attenuation of both airway and alveolar inflammation.13 The findings above suggest that SDCs may be potential biomarkers of airway inflammation. However, nothing is known whether circulating SDC-1 or SDC4 levels are associated with the inflammatory response or airflow limitation in COPD. Therefore, we performed a preliminary clinical study to test the hypothesis that a possible relationship exists between SDCs and lung function, systemic inflammation, as well as risk of exacerbations in COPD patients.
Methods
Subjects and study design
This prospective, observational study was conducted during the period of September 2015 to January 2017 among patients from the Outpatient Department of West China Hospital. The study protocol conforms to the principles of the Declaration of Helsinki and was approved by the Institutional Review Board for Human Studies of West China Hospital of Sichuan University, China. All subjects provided written informed consent. Lung function tests were conducted in all control subjects by standardized methods according to the American Thoracic Society guidelines. COPD was defined based on GOLD strategy paper by the following criteria: a) postbronchodilator FEV1/FVC <70%; b) reversibility of FVC or FEV1 induced by β-agonist (200 mg salbutamol) <12% or 200 ml. Control subjects or patients were excluded if they had pneumonia, active tuberculosis, pulmonary fibrosis, malignant tumor, end-stage renal or liver disease, and an exacerbation within the last 12 weeks prior to recruitment.
Blood collection and analysis
Venous blood was collected from all subjects and immediately centrifuged at 3000 rpm for 10 mins at 4°C. The serum samples were subsequently stored at −80°C until analysis. Levels of SDC-1, SDC-4, and C-reactive protein (CRP) were analyzed using Human Magnetic Luminex Screening Assay (LXSAHM; R&D Systems China Co., Ltd. Shanghai, China) on Bio-Plex 200 detection platform (Bio-Rad, Hercules, CA, USA) by the Department of Laboratory Medicine of West China Hospital strictly according to the manufacturer’s instructions. Technicians performing tests were blinded to the clinical details of the subjects. Blood samples were detected by an SF-3000 blood counter system, and white blood cells were categorized.
Statistical analysis
Comparisons of characteristics between groups were performed by one-way ANOVA or Chi-square tests when appropriate. Distributions of variables such as SDC-1, SDC-4, and CRP were skewed and could not be normalized after log-transformation, so correlations between SDCs and lung function parameters or other inflammatory markers were analyzed using Spearsman’s rho. Multivariate linear regression analysis was conducted to identify the risk factors that determine serum levels of SDCs in the study subjects. Receiver operating curve (ROC) analysis was used to evaluate the threshold value in differentiating disease status. For each ROC, a cutoff point was determined as the value of the parameter that maximized the sum of specificity and sensitivity. All continuous data were presented as mean ± SD, while categorical data were presented as frequency and percent. A two-sided P-value <0.05 was considered statistically significant. All statistical analyses were performed by SPSS 22.0 for Windows (IBM, Chicago, IL, USA).
Results
Subject characteristics
A total of 101 COPD patients and 57 healthy controls were enrolled in this study. All the demographic data and baseline characteristics of the study subjects are summarized in Table 1. Based on the GOLD criteria, 14 patients were categorized as GOLD 1, 37 patients as GOLD 2, 32 patients as GOLD 3, and 18 patients as GOLD 4. As expected, there were significant reductions in FEV1/FVC ratio, FEV1% predicted, and parameters of small airway obstruction (SAO) such as MEF50, MEF25, and MEF75/25 in COPD patients compared to healthy controls. Meanwhile, CRP was found to be increased in COPD patients compared to both control groups.
Table 1.
Clinical character of included subjects
| Variable | Healthy controls (n=57) | COPD (n=101) | P-value | ||
|---|---|---|---|---|---|
| Never smoker (n=37) | Smoker (n=20) | Never smoker (n=23) | Smoker (n=78) | ||
| Age, years | 59.59±13.96 | 59.25±12.37 | 64.17±9.59 | 63.08±8.78 | 0.187 |
| Sex, male/female | 13/24 | 20/0 | 8/15 | 75/3 | <0.001a |
| BMI, kg/m2 | 25.87±3.78 | 24.02±3.17 | 23.84±3.36a | 23.1±3.18b | 0.001a |
| Smoking status | 0.542 | ||||
| Ex-smoker (%) | 13 (65) | 52 (66.7) | |||
| Current smoker (%) | 7 (35) | 26 (33.3) | |||
| Pack-years for ever-smokers | 44.36±32.13 | 38±26.27 | 0.36 | ||
| Frequent exacerbator (≥2 exacerbations per year) (%) | 9 (39.1) | 16 (20.5) | 0.07 | ||
| FEV1/FVC, % | 84±8.19 | 83.27±7.05 | 54.96±9.79b-c | 48.29±14.54b-d | <0.001a |
| FEV1% predicted | 103.98±21.46 | 100.73±16.89 | 55.26±15.56b-c | 52.69±25.08b-c | <0.001a |
| MEF50 | 87.35±36.93 | 88.26±24.7 | 16.73±9.7b-c | 18.76±14.06b-c | <0.001a |
| MEF25 | 90.73±47.1 | 99.08±46.16 | 12.93±8.25b-c | 18.44±12.64b-c | <0.001a |
| MEF75/25 | 81.47±37.83 | 88.85±25.2 | 14.77±8.15b-c | 17.54±12.27b-c | <0.001a |
| WBC, 109/L | 5.39±1.85 | 5.79±1.9 | 6.31±2.7 | 6.51±1.9b | 0.041a |
| CRP, ug/ml | 1.51±1.75 | 1.59±1.86 | 8.11±18.91b | 6.56±13.89b | 0.069 |
Notes: Data are presented as mean ± SD. aSignificant differences among all groups; bsignificantly different compared to healthy never-smoker; csignificantly different compared to healthy smoker; dsignificantly different compared to never-smoking patients.
Abbreviations: BMI, body mass index; CRP, C-reactive protein; FEV1, forced expiratory volume in one second; FVC, forced vital capacity;MEF, maximum expiratory flow; WBC, white blood cells.
Circulating SDCs in COPD and controls
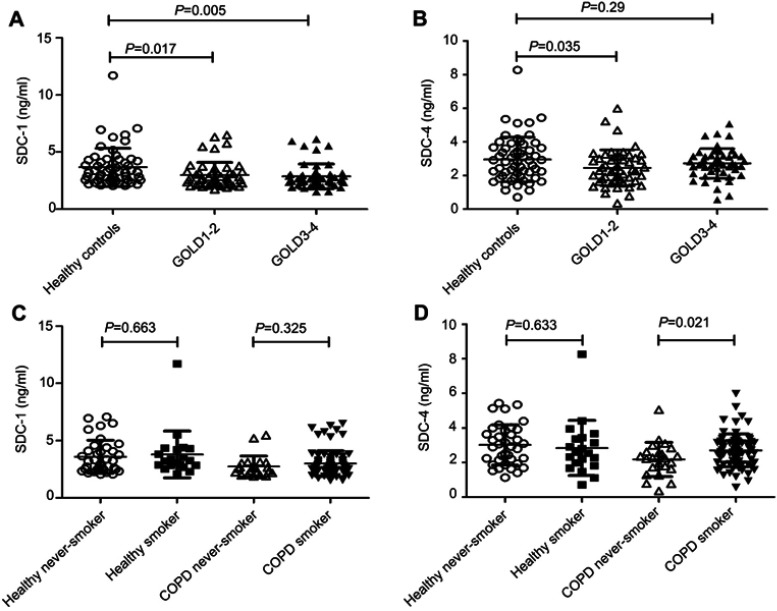
Both serum SDC-1 and SDC-4 levels showed a downward trend in COPD patients compared to healthy controls (P=0.004 and 0.069, respectively). Particularly, SDC-1 was found to be significantly lower in GOLD 3–4 COPD compared with healthy subjects (3.665±1.665 vs 2.876±1.077), while SDC-4 was only decreased significantly in GOLD 1–2 COPD patients (3.665±1.665 vs 2.876±1.077) (Figure 1A and B). However, the effect of smoking on the levels of SDCs was not observed in non-COPD subjects (all P-values>0.05; Figure 1C and D). But for COPD patients, increasing SDC-4 levels were observed in the ever-smoking population (P=0.021; Figure 1D).
Figure 1.
Levels of SDCs in subjects. Both SDC-1 and SDC-4 showed a downward trend in COPD patients (A-B), but the differences in the levels of SDCs between never smoking and ever-smoking controls were not observed (C-D).
Abbreviations: COPD, chronic obstructive pulmonary disease; SDC, syndecan.
Correlations between SDCs and lung function parameters
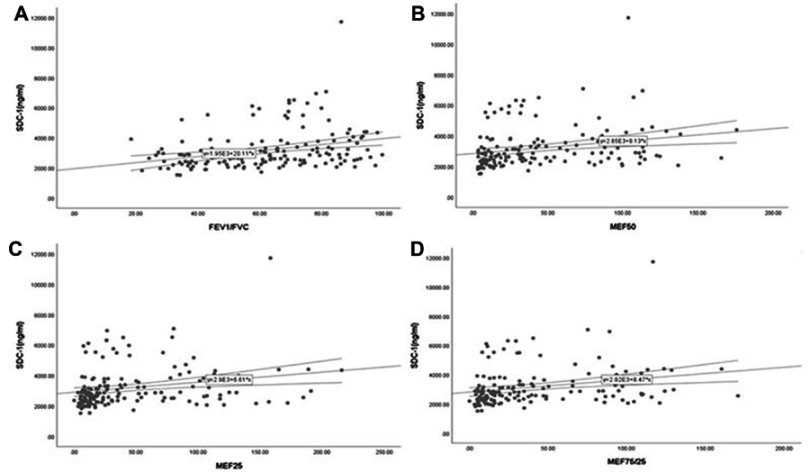
Serum SDC-1 levels in all subjects were correlated positively with the ratio of FEV1/FVC, FEV1% predicted (an index indicating the severity of airflow obstruction), as well as with the parameters of SAO (Table 2 and Figure S1), while no such relationship was found between SDC-4 levels and lung function parameters (all P-values>0.05). However, in COPD patients, SDC-1 levels showed no positive correlation with FEV1% predicted (rho=0.154, P=0.124).
Table 2.
Correlations of SDCs with lung function parameters in all subjects
| SDC-1 | SDC-4 | |||
|---|---|---|---|---|
| rho | P-value | rho | P-value | |
| FEV1/FVC ratio | 0.36 | <0.001 | 0.067 | 0.346 |
| FEV1% predicted | 0.248 | 0.002 | 0.034 | 0.674 |
| MEF50 | 0.337 | <0.001 | 0.072 | 0.371 |
| MEF25 | 0.327 | <0.001 | 0.088 | 0.272 |
| MEF75/25 | 0.339 | <0.001 | 0.024 | 0.768 |
Abbreviations: FEV1, forced expiratory volume in one second; FVC, forced vital capacity; MEF, maximum expiratory flow; SDC, syndecan.
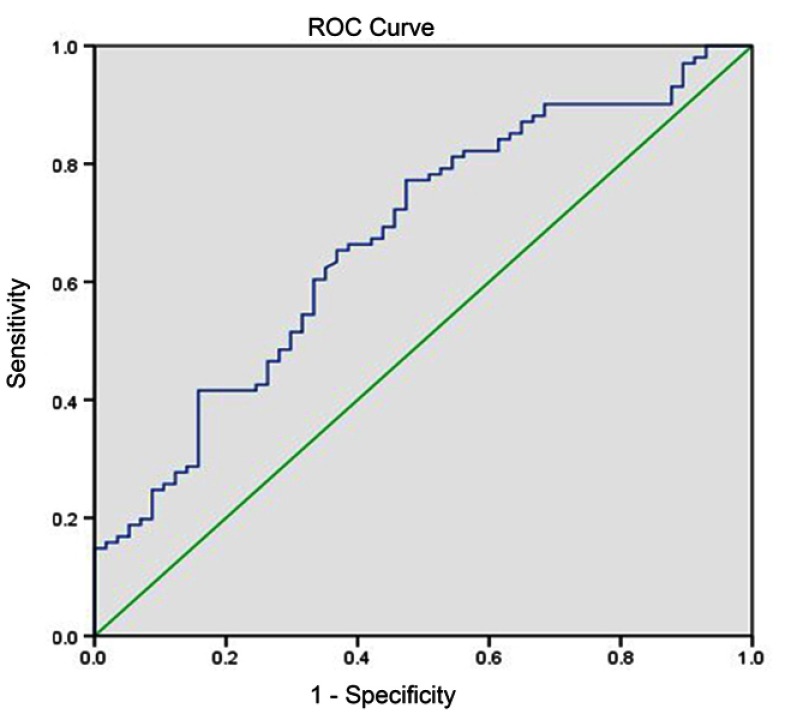
Diagnostic value of SDC-1 for COPD
As SDC-1 levels were significantly different between COPD patients and controls, and correlated positively with lung function parameters, we next explored the possible value of SDC-1 in diagnosing COPD. ROC analyses showed that the area under curve (AUC) of SDC-1 to differentiate COPD was 0.673 (95% CI, 0.587–0.76) (Figure 2). With a cutoff value of 2.88 ng/mL, the sensitivity and specificity were 63.3% and 65.3%, respectively.
Figure 2.
Diagnostic accuracy of serum SDC-1 for COPD. Area under the curves (AUC) was calculated by the trapezoidal rule.
Correlations of SDCs with systemic inflammation and risk of exacerbations
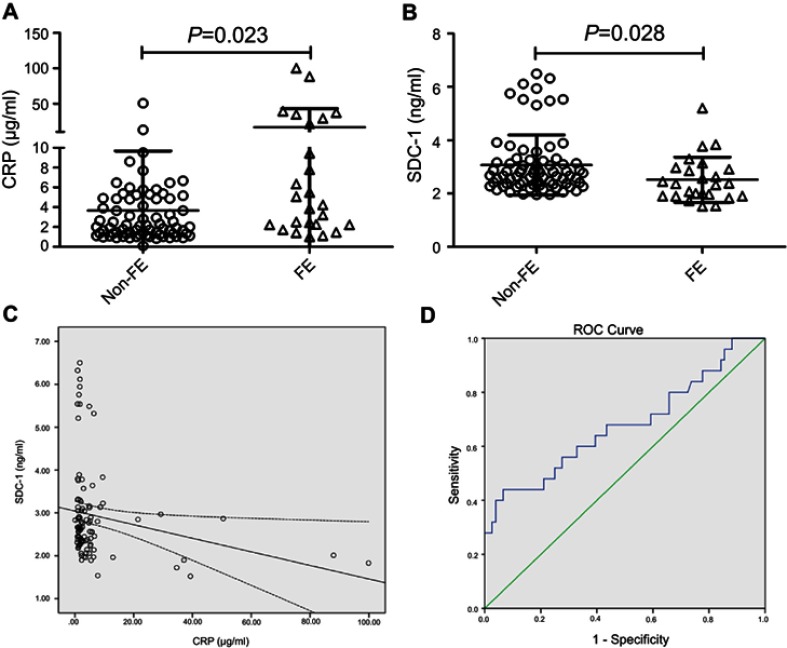
Elevated levels of CRP in individuals with COPD were associated with increased risk of having exacerbations. As expected, our results showed that COPD patients with frequent exacerbations (≥2 exacerbations per year) had higher levels of CRP (Figure 3A). Nevertheless, in frequent exacerbators (FE) of COPD, serum levels of SDC-1 but not SDC-4 were downregulated (P=0.028; Figure 3B). In addition, a negative correlation was observed between serum SDC-1 and CRP in COPD patients (rho=−0.303, P=0.002; Figure 3C). No significant association was found of SDC-4 with CRP in the patient population (rho=−0.096, P=0.349).
Figure 3.
COPD patients with frequent exacerbations (FEs) had higher levels of CRP and lower levels of SDC-1 (A-B). Serum SDC-1 correlated negatively with CRP in COPD patients (C). Diagnostic accuracy of serum SDC-1 to differentiate FE was calculated (D).
Abbreviations: CRP, C-reactive protein; COPD, chronic obstructive pulmonary disease; SDC, syndecan.
Besides, ROC analyses were also performed to test the value of SDC-1 for identifying FEs of COPD. As a result, the AUC of SDC-1 to differentiate FE was 0.678
(95% CI, 0.543–0.813) (Figure 3D). The sensitivity and specificity using a cutoff value of 2.08 ng/mL (determined by the highest Youden index)14 were 44% and 93.4%, respectively.
Multivariate linear analysis
As outlined in Table 3, multivariate linear analysis was performed introducing common confounders such as age, sex, BMI, smoking status, and inflammatory markers WBC and CRP. The results demonstrated that CRP was the independent factor inversely associated with the serum levels of SDC-1.
Table 3.
Multivariate linear analysis with circulating SDC-1 as dependent variable
| β | P-value | |
|---|---|---|
| Age | 0.015 | 0.856 |
| Sex | −0.106 | 0.318 |
| BMI | 0.107 | 0.204 |
| Smoking status | −0.044 | 0.668 |
| WBC, 109/L | −0.09 | 0.285 |
| CRP, ug/ml | −0.175 | 0.031 |
Abbreviations: BMI, body mass index; CRP, C-reactive protein; WBC, white blood cells.
Discussion
The main findings of the present study demonstrated that decreased levels of circulating SDC-1, but not SDC-4, have significant associations with lung function decline and SAO. Besides, compared to patients with less frequent exacerbations, SDC-1 was found to be significantly downregulated in FEs of COPD, also with a high specificity to identify this subgroup. Furthermore, in the patient population, only SDC-1 levels showed negative correlations with CRP, an important biomarker of systemic inflammation and risk factor of having exacerbations. To the best of our knowledge, this is the first retrospective cohort study investigating the relationship between SDCs as inflammatory biomarkers and COPD.
SDC-1 represents a cell surface proteoglycan that can be modulated by many inflammatory mediators in vitro and also by tissue injury and inflammatory conditions in vivo.15,16 Relevant studies identified the activation of SDC-1 expression and shedding to be generally a protective mechanism that may attenuate inflammatory responses. It has been indicated that inducers of allergic lung inflammation promoted SDC-1 shedding into the airway, while knockout of SDC-1 exaggerated the allergic airway inflammation. In addition, administering purified SDC-1 could suppress not only allergen-induced lung inflammation, but also limit lung inflammation and injury during influenza infection.17,18 Nevertheless, a more recent paper reported that in the smoke-induced inflamed bronchial environment, shed forms of SDC-1 in bronchoalveolar lavage fluid would prolong stability and sustain activities of inflammatory mediator proteins.19 Thus, it is still undetermined whether or to what extent can SDC-1 reflect the inflammatory process in COPD. In the present study, circulating SDC-1 levels were significantly reduced in COPD patients, independent of smoking status, and inversely correlated with the levels of CRP, an acute-phase protein synthesized in response to tissue damage or inflammation. We also performed multivariable linear analysis, which revealed that CRP was the independent factor negatively associated with SDC-1 levels, further validating their association. As circulating CRP levels are elevated in COPD patients and are related to the presence of airflow obstruction, it has been regarded as a valid biomarker of systemic inflammation in COPD. Therefore, the negative correlation between SDC-1 and CRP in COPD patients suggests that serum SDC-1 may function as a biomarker of the level of systematic inflammation in COPD.
The strongest predictor of a patient’s future exacerbation frequency remains the number of exacerbations they have had in the prior year.1 Many studies have revealed that excessive inflammation in response to increased oxidative stress is closely related to COPD exacerbation.20 Moreover, a recent systematic review found CRP to be the only robust biomarker showing consistently elevated levels in AECOPD compared with control groups,21 and circulating CRP for AECOPD during the past year was a better risk factor for predicting readmission than sputum inflammatory markers.4,22 Consistent with the previous research, our results showed higher levels of serum CRP in FEs. Importantly, differential expression of SDC-1 was also observed between infrequent and FEs, implying that COPD exacerbation risk might be associated with lower SDC-1 levels. When a threshold value of 2.08 ng/mL was used, SDC-1 had a satisfactory specificity differentiating FEs. Thus, apart from reflecting systematic inflammation, it may be of great potential for SDC-1 to predict COPD exacerbation.
In the present study, we observed that serum SDC-1 levels in COPD patients categorized GOLD 3–4 were particularly downregulated, implying that serum SDC-1 levels may be negatively associated with the severity of disease. To further validate this hypothesis, correlation analyses between SDC-1 levels and lung function parameters were performed, and positive associations of SDC-1 with FEV1/FVC and SAO parameters were found. However, circulating SDC-1 was neither satisfying as a diagnostic marker nor related to FEV1% predicted in COPD patients, indicating that SDC-1 may participate in the development of airflow obstruction but play little role in the disease severity of COPD. The underlying mechanisms of SDC-1 participating in the pathogenesis of COPD require further validation, but the results of the present study would be explained by some indirect clues linking SDC-1 with this disease. It has been shown that the re-epithelialization in tumors might be associated with an increase in the expression (and shedding) of SDC-1, whereas the markers of epithelial–mesenchymal transition (EMT) like Twist were associated with the downregulation of SDC-1.23,24 While numerous studies have revealed that the β-catenin-Snail1-Twist transcription factor cluster was upregulated in COPD and their expression was closely related to both EMT activity and airway obstruction,25 the reduced expression of SDC-1 in COPD may be associated with the enhanced EMT process.
Our study has several limitations. First, the present study was a retrospective analysis which would be affected by the common shortcomings of these types of studies. For example, causality between parameters studied could not be defined, which requires more prospective or in vitro studies to elucidate the potential mechanisms under the findings. Second, due to the rigorous inclusion criteria, only 158 subjects were enrolled, limiting our further investigation on how SDC-1 correlates with lung function and inflammation in different phenotypes of COPD. As for the same reason, although statistically significant changes of SDC-1 levels were observed among COPD groups, magnitude difference was not high (fold-change<2), so it would be necessary to verify our findings in large cohorts of patients. Besides, we selected a population without certain comorbidities, which could arguably result in selection bias. Third, only CRP was analyzed as the indicator of systemic inflammation. It would be better to further investigate the relationship between SDC-1 and inflammatory responses in COPD with a panel of biomarkers. Last, future research should also comprehensively analyze the expression as well as shedding of SDC-1 in different sample types (eg, lung tissue, sputum, bronchoalveolar lavage fluid (BALF), peripheral blood).
In summary, the present study suggests the possibility of circulating SDC-1 level as a novel inflammatory biomarker associated with lung function and systemic inflammation in patients with COPD, which may be also useful to identify the risk of COPD exacerbation. However, further prospectively designed and experimental studies should be performed to clarify the influences of SDC-1 on the pathogenesis and outcomes of COPD.
Supplementary material
Serum SDC-1 correlated positively with the ratio of FEV1/FVC (A) and the parameters of small airway obstruction (SAO) (B-D) in all subjects.
Abbreviations: FEV1, forced expiratory volume in one second; FVC, forced vital capacity; SDC, syndecan.
Acknowledgments
This work was supported by National Key Research and Development Program (2016YFC0903600 and 2016YFC1304500), National Natural Science Foundation of China (81470236, 81670038 and 81830001), 1·3·5 Project for Disciplines of Excellence, West China Hospital, Sichuan University (2018HXFH017) and Health and Family Planning Commission of Sichuan Province (17PJ009). The funders had no role in study design, data collection or analysis, decision to publish, and manuscript preparation. We also thank Dr Haiqiao Wu for providing assistance in data collection.
Author contributions
Diandian Li, Yanqiu Wu, Shujin Guo, Lei Chen, and Fuqiang Wen designed this research, and all authors contributed toward subjects recruit, data collection, statistical analysis, drafting, and critically revising the paper, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Vogelmeier CF, Criner GJ, Martinez FJ, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive lung disease 2017 report. GOLD Executive Summary. Am J Respir Crit Care Med. 2017;195(5):557–582. doi: 10.1164/rccm.201701-0218PP [DOI] [PubMed] [Google Scholar]
- 2.Fabbri LM, Rabe KF. From COPD to chronic systemic inflammatory syndrome? Lancet. 2007;370(9589):797–799. doi: 10.1016/S0140-6736(07)61383-X [DOI] [PubMed] [Google Scholar]
- 3.Ferrari R, Tanni SE, Caram LM, Correa C, Correa CR, Godoy I. Three-year follow-up of interleukin 6 and C-reactive protein in chronic obstructive pulmonary disease. Respir Res. 2013;14:24. doi: 10.1186/1465-9921-14-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jing Z, Chun C, Ning S, Hong Z, Bei H, Wan-Zhen Y. Systemic inflammatory marker CRP was better predictor of readmission for AECOPD than sputum inflammatory markers. Arch Bronconeumol. 2016;52(3):138–144. doi: 10.1016/j.arbres.2015.01.011 [DOI] [PubMed] [Google Scholar]
- 5.Hogg JC. A pathologist’s view of airway obstruction in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2012;186(5):v–vii. doi: 10.1164/rccm.201206-1130ED [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stanojkovic I, Kotur-Stevuljevic J, Milenkovic B, et al. Pulmonary function, oxidative stress and inflammatory markers in severe COPD exacerbation. Respir Med. 2011;105(Suppl 1):S31–S37. doi: 10.1016/S0954-6111(11)70008-7 [DOI] [PubMed] [Google Scholar]
- 7.Conti V, Corbi G, Manzo V, et al. SIRT1 activity in peripheral blood mononuclear cells correlates with altered lung function in patients with chronic obstructive pulmonary disease. Oxid Med Cell Longev. 2018;2018:9391261. doi: 10.1155/2018/9391261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kufareva I. Chemokines and their receptors: insights from molecular modeling and crystallography. Curr Opin Pharmacol. 2016;30:27–37. doi: 10.1016/j.coph.2016.07.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Agere SA, Kim EY, Akhtar N, Ahmed S. Syndecans in chronic inflammatory and autoimmune diseases: pathological insights and therapeutic opportunities. J Cell Physiol. 2018;233(9):6346–6358. doi: 10.1002/jcp.26388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li Q, Park PW, Wilson CL, Parks WC. Matrilysin shedding of syndecan-1 regulates chemokine mobilization and transepithelial efflux of neutrophils in acute lung injury. Cell. 2002;111(5):635–646. doi: 10.1016/s0092-8674(02)01079-6 [DOI] [PubMed] [Google Scholar]
- 11.Park PW, Pier GB, Hinkes MT, Bernfield M. Exploitation of syndecan-1 shedding by pseudomonas aeruginosa enhances virulence. Nature. 2001;411(6833):98–102. doi: 10.1038/35075100 [DOI] [PubMed] [Google Scholar]
- 12.Luo L, Feng S, Wu Y, Su Y, Jing F, Yi Q. Serum levels of syndecan-1 in patients with kawasaki disease. Pediatr Infect Dis J. 2019;38(1):89–94. doi: 10.1097/INF.0000000000002047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Santoso A, Kikuchi T, Tode N, et al. Syndecan 4 mediates Nrf2-dependent expansion of bronchiolar progenitors that protect against lung inflammation. Mol Ther. 2016;24(1):41–52. doi: 10.1038/mt.2015.153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee KS, Kim HR, Kwak S, et al. Association between elevated pleural interleukin-33 levels and tuberculous pleurisy. Ann Lab Med. 2013;33(1):45–51. doi: 10.3343/alm.2013.33.1.45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sanchez-Pedrosa G, Vara Ameigeiras E, Casanova Barea J, Rancan L, Simon Adiego CM, Garutti Martinez I. Role of surgical manipulation in lung inflammatory response in a model of lung resection surgery. Interact Cardiovasc Thorac Surg. 2018;27:870–877. doi: 10.1093/icvts/ivy198 [DOI] [PubMed] [Google Scholar]
- 16.Lu Z, Song N, Shen B, et al. Syndecan-1 shedding inhibition to protect against ischemic acute kidney injury through HGF target signaling pathway. Transplantation. 2018;102(7):e331–e344. doi: 10.1097/TP.0000000000002170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xu J, Park PW, Kheradmand F, Corry DB. Endogenous attenuation of allergic lung inflammation by syndecan-1. J Immunol. 2005;174(9):5758–5765. doi: 10.4049/jimmunol.174.9.5758 [DOI] [PubMed] [Google Scholar]
- 18.Brauer R, Ge L, Schlesinger SY, et al. Syndecan-1 attenuates lung injury during influenza infection by potentiating c-Met signaling to suppress epithelial apoptosis. Am J Respir Crit Care Med. 2016;194(3):333–344. doi: 10.1164/rccm.201509-1878OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lam DC, Chan SC, Mak JC, Freeman C, Ip MS, Shum DK. S-maltoheptaose targets syndecan-bound effectors to reduce smoking-related neutrophilic inflammation. Sci Rep. 2015;5:12945. doi: 10.1038/srep12945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Celli BR, Barnes PJ. Exacerbations of chronic obstructive pulmonary disease. Eur Respir J. 2007;29(6):1224–1238. doi: 10.1183/09031936.00109906 [DOI] [PubMed] [Google Scholar]
- 21.Chen YW, Leung JM, Sin DD. A systematic review of diagnostic biomarkers of COPD exacerbation. PLoS One. 2016;11(7):e0158843. doi: 10.1371/journal.pone.0158843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Crisafulli E, Torres A, Huerta A, et al. C-reactive protein at discharge, diabetes mellitus and >/=1 hospitalization during previous year predict early readmission in patients with acute exacerbation of chronic obstructive pulmonary disease. COPD. 2015;12(3):306–314. doi: 10.3109/15412555.2014.933954 [DOI] [PubMed] [Google Scholar]
- 23.Rautava J, Soukka T, Heikinheimo K, Miettinen PJ, Happonen RP, Jaakkola P. Different mechanisms of syndecan-1 activation through a fibroblast-growth-factor-inducible response element (FiRE) in mucosal and cutaneous wounds. J Dent Res. 2003;82(5):382–387. doi: 10.1177/154405910308200511 [DOI] [PubMed] [Google Scholar]
- 24.Vered M, Dayan D, Yahalom R, et al. Cancer-associated fibroblasts and epithelial-mesenchymal transition in metastatic oral tongue squamous cell carcinoma. Int J Cancer. 2010;127(6):1356–1362. doi: 10.1002/ijc.25358 [DOI] [PubMed] [Google Scholar]
- 25.Mahmood MQ, Walters EH, Shukla SD, et al. beta-catenin, twist and snail: transcriptional regulation of EMT in smokers and COPD, and relation to airflow obstruction. Sci Rep. 2017;7(1):10832. doi: 10.1038/s41598-017-11375-x [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Serum SDC-1 correlated positively with the ratio of FEV1/FVC (A) and the parameters of small airway obstruction (SAO) (B-D) in all subjects.
Abbreviations: FEV1, forced expiratory volume in one second; FVC, forced vital capacity; SDC, syndecan.