Abstract
Transintestinal cholesterol excretion (TICE) is a major route for eliminating cholesterol from the body and a potential therapeutic target for hypercholesterolemia. The underlying mechanism, however, is largely unclear, and its contribution to cholesterol disposal from the body is obscured by the counteracting process of intestinal cholesterol reabsorption. To determine the quantity of TICE independent from its reabsorption, we studied two models of decreased intestinal cholesterol absorption. Cholesterol absorption was inhibited either by ezetimibe or, indirectly, by the genetic inactivation of the intestinal apical sodium-dependent bile acid transporter (ASBT; SLC10A2). Both ezetimibe treatment and Asbt inactivation virtually abrogated fractional cholesterol absorption (from 46% to 4% and 6%, respectively). In both models, fecal neutral sterol excretion and net intestinal cholesterol balance were considerably higher than in control mice (5- and 7-fold, respectively), suggesting that, under physiological conditions, TICE is largely reabsorbed. In addition, the net intestinal cholesterol balance was increased to a similar extent but was not further increased when the models were combined, suggesting that the effect on cholesterol reabsorption was already maximal under either condition alone. On the basis of these findings, we hypothesize that the inhibition of cholesterol (re)absorption combined with stimulating TICE will be most effective in increasing cholesterol disposal.
Keywords: ASBT inhibition, intestinal cholesterol absorption, transintestinal cholesterol excretion, ezetimibe
Atherosclerosis, which can lead to coronary artery disease and cerebrovascular accidents, accounts for approximately 50% of deaths in Westernized countries, and its prevalence is increasing in low- and middle-income countries (1, 2). The etiology of atherosclerosis is complex, and risk factors are both genetic and environmental. Hypercholesterolemia can contribute to the development of atherosclerosis via the accumulation of cholesterol from LDLs in the arterial vessel wall (2, 3). Treatment options for hypercholesterolemia and atherosclerosis include lifestyle modifications (e.g., ceasing to smoke, increasing physical activity, improving diet quality) (4, 5), as well as drugs targeted at cholesterol metabolism. The most widely prescribed class of drugs are statins that inhibit hepatic cholesterol synthesis. Unfortunately, statins reduce the cardiovascular disease risk only by 15% to 37% (6). Novel proprotein convertase subtilsinkexin type 9 inhibitors show promising results but also limitations (7). Therefore, more effective or adjunct treatments are needed for the prevention and treatment of atherosclerosis.
Cholesterol homeostasis in the body encompasses a highly regulated balance between cholesterol intake, de novo synthesis, and disposal, mainly via the feces as neutral sterols (NSs; cholesterol and its metabolites produced by intestinal microbiota) or as bile acids (BAs). BAs are synthetized from cholesterol in the liver, secreted into the bile, and predominantly stored in the gallbladder. Following a meal and gallbladder emptying, BAs are secreted via the bile into the duodenum, where they aid in the absorption of fat, cholesterol, and fat-soluble vitamins. Under physiological conditions, about 95% of intestinal BAs are reabsorbed each cycle, mainly by ileal enterocytes via the apical sodium-dependent bile acid transporter (ASBT; SLC10A2), and transported back to the liver. This mechanism of enterohepatic circulation is tightly regulated by the BA-activated nuclear farnesoid X receptor (FXR) in both the liver and intestine (8).
Inducing the fecal excretion of both NSs and BAs has been used as a strategy to lower plasma cholesterol levels. Blocking cholesterol absorption by inhibiting the main intestinal cholesterol transporter Niemann-Pick C1-like 1 (NPC1L1) increases fecal cholesterol excretion and hepatic LDL-receptor expression and is used as adjunct therapy to statins to further reduce plasma LDL-cholesterol (LDL-C) levels and improve cardiovascular outcomes (9). Increasing cholesterol excretion from the body can also be achieved by targeting BA homeostasis. BA sequestrants bind BAs inside the intestinal lumen, thereby preventing their reabsorption, ultimately resulting in increased fecal BA excretion and a compensatory increase in BA synthesis from cholesterol (10). BA sequestrants have been shown to effectively lower plasma LDL-C levels in animal models as well as in humans (11). ASBT inhibition works via a similar mechanism and has also been shown to reduce hypercholesterolemia and atherosclerosis in several animal models (12–15). The inhibition of ASBT reduces the reabsorption of BAs. Like BA sequestrants, this increases their fecal excretion, which is compensated by increased hepatic synthesis from cholesterol. Interrupting the enterohepatic circulation by inhibiting ASBT decreases the BA pool (i.e., the total amount of BAs present in the enterohepatic circulation) because the induction of synthesis cannot completely compensate for the increased fecal loss (16). This results in a decreased availability of BAs in the intestinal lumen for the solubilization of cholesterol, thereby lowering intestinal cholesterol absorption (17). Therefore, in contrast to ezetimibe, which directly inhibits intestinal cholesterol absorption, ASBT inactivation indirectly lowers absorption of cholesterol through a reduction of the BA pool.
A major nonbiliary pathway that contributes to the fecal excretion of cholesterol has recently been identified. This pathway, known as transintestinal cholesterol excretion (TICE), is present both in mice and humans (18, 19). The molecular mechanism underlying TICE has not been fully elucidated. However, TICE is at least partly dependent on cholesterol transport by ABCG members 5 and 8 (20–22). Originally, Van der Velde et al. quantified TICE directly in intestinal perfusion studies (23, 24). TICE was also estimated in models with impaired biliary cholesterol secretion such as the Abcg8 or multidrug-resistant protein 2 knockout mouse (23). This study and others indirectly calculated TICE by subtracting dietary and biliary (or only dietary in the case of impaired biliary secretion) input from fecal NS output [reviewed in (25, 26)]. In models of impaired biliary cholesterol secretion, such as the multidrug-resistant protein 2 knockout mouse, fractional cholesterol absorption is still high, with studies showing no reduction compared with WT controls (∼50% absorption in both genotypes) (27) to a reduction from 70% to 40% (28). Therefore, the calculated TICE in these studies yields a minimum estimation, as it is unclear to what degree the reabsorption of transintestinally excreted cholesterol contributed to fecal NS excretion. The notion that the reabsorption of TICE occurs could be hypothesized on the basis of experiments with ezetimibe, an NPC1L1 inhibitor, which increased fecal NS excretion beyond biliary and dietary input and potentiated the effects on calculated TICE by intestinal FXR activation (19, 22, 29, 30). While various conditions, including high-fat diet feeding, LXR activation, and (intestinal) FXR activation have been implied to affect TICE, the possible role of the reabsorption of cholesterol originating from TICE has not been addressed rigorously (21–23).
In the current study, we investigated the contribution of cholesterol (re)absorption of intestinally excreted cholesterol by using two models of impaired cholesterol (re)absorption. First, we inhibited intestinal cholesterol (re)absorption by using ezetimibe, which inhibits NPC1L1. Second, we used Asbt−/− mice that display a partial impairment in cholesterol (re)absorption through the reduction of the BA pool (17). In both models, we quantitated cholesterol fluxes and measured fractional cholesterol absorption. Finally, we combined both models to determine the combined effect of ezetimibe and ASBT inhibition on cholesterol disposal from the body.
MATERIALS AND METHODS
Animals
Asbt−/− mice and WT littermates on a C57BL/6 background were originally generated by P. A. Dawson (Emory University, Atlanta, GA) and bred at the University Medical Center Groningen animal facility. While there are established differences in sterol metabolism between male and female mice (31), most studies on intestinal cholesterol fluxes were performed on male mice with a C57BL/6 background (19–24, 29, 32). To be able to best relate our results to previously published studies, only male mice (aged 10–18 weeks) were used. Mice were conventionally housed in individual cages in a temperature- and light-controlled facility with a 12-h light-dark cycle. The mice had ad libitum access to water and maintenance laboratory chow (macronutrient ratio as percentage of total calories; fat: 7.5%, proteins: 17.5%, and carbohydrates: 75%) containing 0.008% cholesterol (RM1 FG; Special Diet Services, Witham, UK) with or without ezetimibe (0.005%; 50 mg/kg chow) (Ezetrol; University Medical Center Groningen).
Animal experiments were approved by the University of Groningen Ethics Committee for Animal Experiments of the University of Groningen. All experiments were performed in accordance with relevant guidelines and regulations (including laboratory and biosafety regulations).
Cholesterol flux measurements
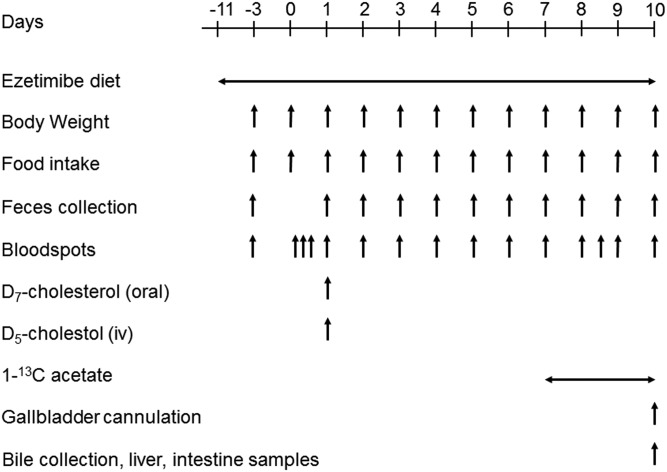
Mice received the ezetimibe-enriched diet for 3 weeks. Cholesterol fluxes were measured in the last 10 days by using a dual stable isotope tracer method as previously described (33). The experimental setup is shown in Fig. 1. Three days prior to the start of the experiment, bloodspots and 24 h feces were collected for baseline measurements, and food intake and body weight were measured. On day 0, the mice were anesthetized with isoflurane and given a retro-orbital injection of 0.3 mg D5-cholesterol (Medical Isotopes Inc., Pelham, NH) dissolved in 150 μl Intralipid 20% (Fresenius Kabi, Den Bosch, the Netherlands) and an oral gavage of 0.6 mg D7-cholesterol (Cambridge Isotope Laboratories, Inc., Andover, MA) dissolved in 200 μl medium-chain triglyceride oil. Bloodspots were collected at 3, 6, 12, 24, 48, 72, 96, 120, 144, and 168 h after labeled cholesterol administration. At 168 h (day 8), mice received water containing 2% 1-13Cacetate until termination, and bloodspots were collected 24, 32, 48, and 72 h after starting the 1-13Cacetate. Body weight and food intake were determined, and feces were collected daily from day 0 to day 10 (Fig. 1).
Fig. 1.
Experimental schedule. Adapted from (21).
On day 10, the mice were anesthetized by an intraperitoneal injection of a mixture of Hypnorm (fentanyl/fluanisone; 1 ml/kg) and diazepam (10 mg/kg). The gallbladder was cannulated early in the light phase (at 9:00 AM) as previously described (34). Bile collected in the first 5 min was discarded to avoid collecting concentrated bile. After this first 5 min, bile was collected for 20 min in preweighed tubes, with the mice placed in a humidified incubator (37°C) to maintain body temperature. Blood was obtained via cardiac puncture. The small intestines were flushed with ice-cold PBS containing a protease inhibitor (cOmplete; Roche Diagnostics, Mannheim, Germany) and cut in three segments of equal length; the middle piece from each segment was excised for gene analysis. All intestinal segments were immediately snap-frozen in liquid nitrogen.
BA and NS measurements
NSs (cholesterol and its bacterial metabolites in fecal samples) were extracted from 50 mg air-dried, ground fecal samples as described by Ronda et al. (33). Briefly, feces were heated for 2 h at 80°C with a mixture of 1 M sodium hydroxide and methanol (1:3). NSs were then extracted two times with 2 ml petroleum ether and derivatized with N,O-bis(trimethylsilyl)trifluoroacetamide (BSTFA)-pyridine-trimethylchlorosilane (TMCS) (5:5:0.1). BAs were extracted from feces with Sep-Pak C-18 columns, methylated with methanol-acetyl chloride (20:1), and derivatized with BSTFA-pyridine-TMCS (5:5:0.1). Both NSs and BAs were measured by GC as previously described (35). The total amount of BAs or NSs was calculated as the sum of the individual species.
For biliary BA measurements, bile samples were diluted 1,000-fold with Milli-Q water. Samples were centrifuged at 15,800 g, and the supernatant was poured into a clean glass tube. The fluid was evaporated under nitrogen at 40°C. Before measuring, samples were reconstituted in 200 µl 50% methanol in water, vortexed for 60 s, and centrifuged for 3 min at 1,800 g. The supernatant was transferred into a 0.2 µm spin filter and centrifuged at 2,000 g for 10 min. After filtering, the samples were transferred into vials and analyzed (10 µl injection volume). For the quantitative determination of BAs, we used a Nexera X2 ultra-high-performance LC system (Shimadzu, Kyoto, Japan) coupled to a QTRAP 4500 MD triple-quadrupole mass spectrometer (SCIEX, Framingham, MA). The LC/MS/MS system is controlled by Analyst MD 1.6.2 software.
Biliary lipids were extracted from 15 μl of bile according to Bligh and Dyer (36). Biliary cholesterol was then derivatized with BSTFA-pyridine-TMCS (5:5:0.1) for GC measurement (33).
Fecal NS and BA excretion and dietary cholesterol intake were similar among all days. Data displayed in the figures and used to calculate the intestinal cholesterol balance represent measured values for the last 24 h (day 10). Net nonhepatobiliary cholesterol excretion was calculated as [fecal NS output − (dietary cholesterol intake + hepatobiliary secretion)]. Cholesterol synthesis and pool size were calculated as described in Ronda et al. (33).
Hepatic and plasma lipids
Livers were mechanically ground in liquid nitrogen. Liver lipids were extracted from 15% homogenates in PBS according to Bligh and Dyer (36). Liver total and free cholesterol and triglyceride levels were then determined using commercially available reagents (DiaSys Diagnostic Systems, Holzheim, Germany; Roche Diagnostics). Plasma triglycerides, total cholesterol, and free cholesterol were determined spectrophotometrically using the same kits. For plasma lipoprotein measurements, blood from individual mice was pooled for each experimental group. Plasma lipoproteins were fractionated using fast-protein LC on a Superose 6 10/300 GL column (GE Healthcare, Uppsala, Sweden). Cholesterol and triglyceride concentrations of fractions were determined using commercially available reagents (DiaSys Diagnostic Systems and Roche Diagnostics).
Gene expression analysis
Gene expression analysis was performed in the liver and duodenum. Total RNA was isolated with TRI Reagent (Sigma-Aldrich, St. Louis, MO) and quantified by NanoDrop (NanoDrop Technologies, Wilmington, DE). cDNA synthesis was performed from 1 μg total RNA. Primers were designed with Primer-BLAST and optimized for use with SYBR Green Master Mix (Roche Diagnostics) (maximum product size: 150 nucleotides). Real-time quantitative PCR analysis was performed on a StepOnePlus Real-Time PCR System (Thermo Fisher Scientific, Darmstadt, Germany). Gene expression levels were normalized to 36b4. Results were quantified using the comparative Ct method.
Statistical analyses
Unless otherwise stated, data are presented as Tukey plots, where boxes represent the median with interquartile range and whiskers extend to the largest value or 1.5 times the interquartile range if the largest value exceeds that. Statistical analyses were performed, and graphs were created using GraphPad Prism 6 (GraphPad Software, La Jolla, CA). Differences between groups were assessed by two-way ANOVA using Tukey’s post hoc test. Significance is indicated as *P < 0.05, **P < 0.01, ***P < 0.001.
RESULTS
Assessment of cholesterol (re)absorption in ezetimibe-treated mice
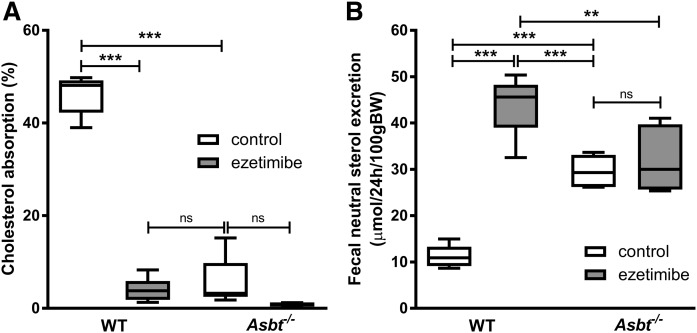
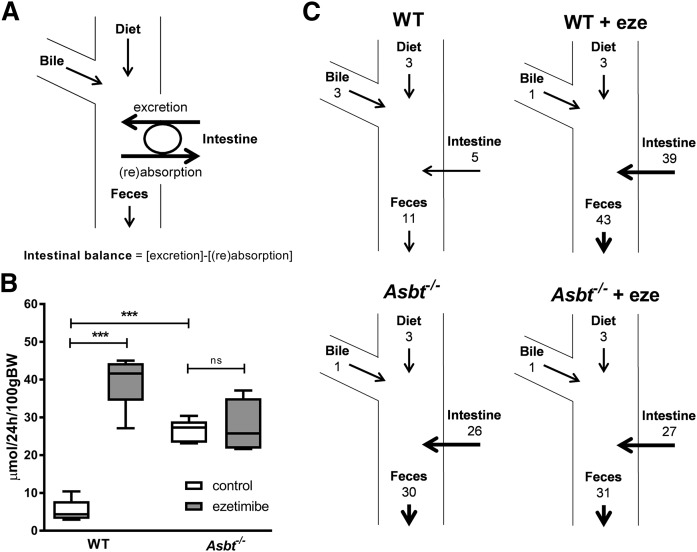
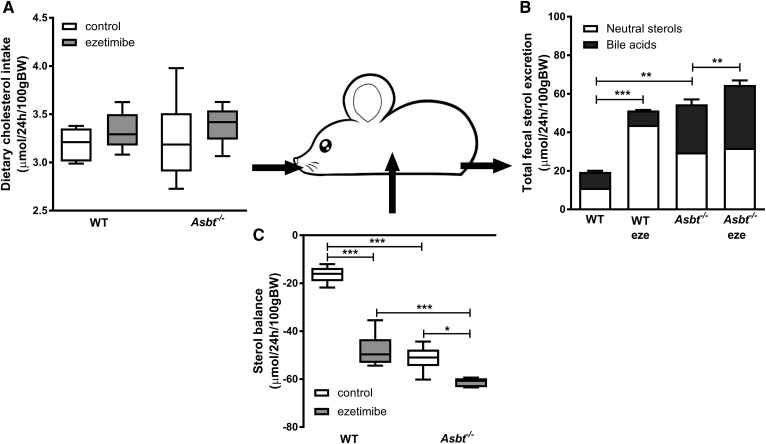
We aimed to estimate the amount of cholesterol entering the intestine via TICE. To avoid interfering with potential intestinal reabsorption, we first applied ezetimibe and determined fractional cholesterol absorption using a dual stable isotope labeling approach (Fig. 2A). Ezetimibe prevents intestinal cholesterol absorption by inhibiting the internalization of the cholesterol transporter NPC1L1, which is required for intestinal cholesterol absorption in the small intestinal epithelium (37). Ezetimibe treatment virtually abrogated fractional cholesterol absorption (from 46% in untreated controls to 4% upon ezetimibe treatment, P < 0.001; Fig. 2A). In line with earlier studies (22), ezetimibe treatment increased fecal NS excretion 4-fold in WT mice (Fig. 2B). Theoretically, there are three mechanisms possible for this increase in fecal NSs 1) increased influx of cholesterol into the intestine (either via the bile, the diet, or TICE), 2) decreased intestinal (re)absorption, or 3) a combination of these. Because cholesterol absorption is virtually abrogated by ezetimibe treatment, cholesterol (re)absorption can be considered minimal, leaving the three remaining fluxes as possibly causing the increase in fecal NSs: biliary secretion, dietary cholesterol intake, and/or TICE (Fig. 3A). The resultant flux of excretion (i.e., TICE) minus (re)absorption was defined as net intestinal (cholesterol) balance. (Fig. 3A). Based on the difference between fecal NS excretion and the dietary and biliary cholesterol influx into the intestine, the net intestinal balance in ezetimibe-treated mice was estimated at 39 μmol/24 h/100 g body weight (BW) (Fig. 3B, C). The estimated net intestinal cholesterol balance thereby largely exceeds the biliary and dietary influx of cholesterol into the intestine. These data indicate that the profound increase in fecal NSs is for the most part due to TICE, which is not reabsorbed upon ezetimibe treatment.
Fig. 2.
Intestinal cholesterol absorption and fecal NS excretion in WT and Asbt−/− mice with and without ezetimibe treatment. A: Fractional cholesterol absorption measured by stable dual isotope method. B: Total NS excretion in feces. n = 5-6 per group.
Fig. 3.
Proposed model for intestinal cholesterol fluxes and calculated net intestinal cholesterol balance for WT and Asbt−/− mice with and without ezetimibe treatment. Net intestinal (cholesterol) balance represents [intestinal excretion (i.e., TICE)] − [intestinal (re)absorption] (A), calculated net intestinal cholesterol balance (B), and resultant cholesterol fluxes according to the proposed model (C). Values in μmol/24 h/100 g BW; n = 5–6 per group.
Assessment of cholesterol (re)absorption in Asbt−/− mice
Theoretically, the results obtained in ezetimibe-treated mice could be specific for this mechanism of inhibition of cholesterol absorption and thereby not generalizable to other conditions of decreased cholesterol (re)absorption. We therefore performed similar experiments in another mouse model of decreased cholesterol absorption and increased fecal NS excretion, the Asbt−/− mice. The fractional cholesterol absorption in Asbt−/− mice was strongly decreased to a similar level as that of ezetimibe-treated WT mice (6% vs. 4%, P = ns; Fig. 2A). In agreement with previous reports, Asbt−/− mice had a 3-fold increased fecal NS excretion compared with WT controls (P < 0.001; Fig. 2B) (17). Under these conditions of virtually no cholesterol (re)absorption, the net intestinal cholesterol balance was 26 μmol/24 h/100 g BW, slightly lower but in the same range as the intestinal cholesterol balance in ezetimibe-treated WT mice (P < 0.01; Fig. 3B, C). TICE was several-fold larger than the biliary and dietary cholesterol influx into the intestine and, again, the increased fecal NS fraction could largely be attributed to nonreabsorbed cholesterol originating from TICE.
We then investigated whether combining the two mechanisms of inhibiting (re)absorption would further affect these cholesterol fluxes across the intestine. Treating Asbt−/− mice with ezetimibe, however, did not result in a significant (further) reduction of cholesterol absorption compared with untreated Asbt−/− mice (1% vs. 6%, P = ns; Fig. 2A) or affect the fecal NS excretion in Asbt−/− mice (Fig. 2B), the biliary or dietary cholesterol influx, or the calculated net intestinal cholesterol balance (Fig. 3B, C). This observation establishes impaired cholesterol (re)absorption as the mechanism underlying the increased fecal NS excretion in (untreated) Asbt−/− mice.
Mechanism of decreased cholesterol (re)absorption in Asbt−/− mice
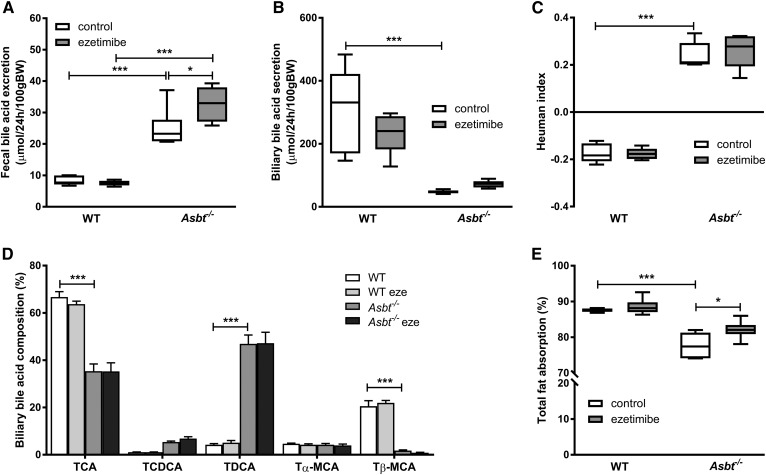
For ezetimibe, the mechanism of inhibition of cholesterol (re)absorption has been directly related to the inhibition of NPC1L1, the main protein responsible for cholesterol absorption (38). For Asbt−/− inactivation, however, the mechanism of decreased cholesterol (re)absorption has been less clear (39). Intestinal cholesterol absorption is strongly dependent on the intestinal availability of (hydrophobic) BAs (40). Decreased biliary BA secretion, due to increased intestinal BA loss and the contraction of the BA pool, could underlie the decreased cholesterol (re)absorption. We therefore determined whether the interrupted enterohepatic circulation in Asbt−/− mice quantitatively and/or qualitatively affected biliary BA secretion and fecal BA excretion. Fecal BA excretion was about 3-fold higher in Asbt−/− mice compared with WT mice (25 vs. 8 μmol/24 h/100 g BW, P < 0.001; Fig. 4A). At the same time, the mRNA level of cholesterol 7 α-hydroxylase (Cyp7a1), the rate-limiting enzyme in the conversion of cholesterol to BAs, was increased 7-fold in Asbt−/− mice compared with WT mice (supplemental Fig. S1). Ezetimibe treatment did not change fecal BA excretion in WT mice but slightly increased BA excretion in Asbt−/− mice (33 vs. 25 μmol/24 h/100 g BW, P < 0.05; Fig. 4A). In line with the interruption of the enterohepatic circulation of BAs and the subsequent contraction of the BA pool, the biliary secretion of BAs was strongly decreased in Asbt−/− mice compared with WT controls (−86%, P < 0.001; Fig. 4B). Ezetimibe did not significantly affect total biliary BA secretion in WT mice or Asbt−/− mice (Fig. 4B). The composition of biliary BAs was more hydrophobic in Asbt−/− mice compared with controls, as quantified by an increased Heuman hydrophobicity index (+0.2 vs. −0.2, P < 0.001; Fig. 4C) (41). The increase in hydrophobicity could be attributed to a fractional increase of taurodeoxycholic acid (TDCA) (47% vs. 4%, P < 0.001 Fig. 4D) and subsequent decrease in taurocholic acid (TCA) (35% vs. 67%, P < 0.001; Fig. 4D) and tauro-β-muricholic acid (TβMCA) (2% vs. 21%, P < 0.001; Fig. 4D) in Asbt−/− mice compared with WT mice. Ezetimibe did not affect fecal BA composition (data not shown), biliary hydrophobicity (Fig. 4C), or biliary BA profile (Fig. 4D) in either WT or Asbt−/− mice .
Fig. 4.
Changes in fecal and biliary BAs and intestinal fat absorption upon ezetimibe treatment and Asbt deletion in mice. Total fecal BA excretion (A), total biliary BA secretion (B), biliary hydrophobicity index based on Heuman values (C), biliary BA composition (D), and intestinal fatty acid absorption (E). n = 5–6 per group.
The absorption of not only cholesterol but also dietary fatty acids was decreased in Asbt−/− mice, and this was slightly ameliorated by ezetimibe (Fig. 4E). Together, these findings indicate that the abrogated intestinal cholesterol absorption in Asbt−/− mice primarily resulted from strongly reduced biliary BA secretion. Apparently, the decreased biliary BA secretion could not be compensated for by a more hydrophobic BA composition, despite the notion that hydrophobic BAs are more effective in aiding the micellar solubilization and subsequent absorption of cholesterol (40, 42).
Intestinal and hepatic mRNA expression of genes involved in cholesterol homeostasis
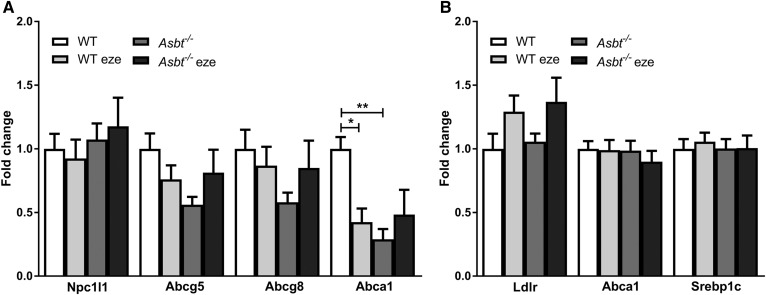
Theoretically, the decreased cholesterol absorption in Asbt−/− mice could be due to the downregulation of Npc1l1 expression in the duodenum, but the unaffected steady-state mRNA levels did not support this possibility (Fig. 5A). A reduction in cholesterol absorption can affect intracellular cholesterol concentrations, which is sensed by the LXR. Therefore, we measured the expression of LXR target genes, Abca1 and Abcg5/8. Abcg5 and Abcg8 promote cholesterol efflux from the cell and are known to be crucial for TICE (25, 43). Abca1 was decreased in WT ezetimibe-treated mice compared with WT controls and in Asbt−/− mice compared with WT controls (Fig. 5A). Abcg5/8 showed a similar trend to Abca1, but the difference did not reach statistical significance (Fig. 5A).
Fig. 5.
Expression of genes related to cholesterol homeostasis in WT and Asbt−/− mice with and without ezetimibe treatment. Duodenal expression of genes related to cellular cholesterol homeostasis (A) and hepatic expression of LXR target genes (B). n = 5–6 per group.
To assess the consequences of decreased intestinal cholesterol (re)absorption and increased BA synthesis on hepatic cholesterol homeostasis, we also measured LXR target genes in the liver (Fig. 5B). Levels of mRNA of Ldlr1, Abca1, and the sterol regulatory element-binding protein 1c were not statistically different.
Effects of ezetimibe treatment and Asbt inactivation on total sterol excretion and cholesterol synthesis
The disposal of cholesterol from the body is achieved via excretion either as NSs or, after conversion, as BAs. We calculated the total sterol balance from the total fecal sterol output and dietary sterol input without correcting for de novo synthesis, which will be assessed later. As this model includes all cholesterol fluxes into the intestinal lumen, it represents a total sterol input-output balance over the intestine. Dietary sterol input (composed of dietary cholesterol ingestion) was similar across the groups (Fig. 6A). Total fecal sterol output, calculated as the sum of NS and BA output, was elevated in Asbt−/− control and WT ezetimibe-treated mice to a similar degree compared with WT controls (Fig. 6B). In Asbt−/− mice, ezetimibe treatment further augmented total sterol excretion in the form of BAs (by ∼17%, P < 0.01; Fig. 6B). All mice displayed a negative intestinal sterol balance, implying that sterol output was greater than input (Fig. 6C). Total intestinal sterol balance in Asbt−/− and ezetimibe-treated WT mice was similarly negative (implying more disposal than input), although Asbt−/− mice excreted more sterols in the form of BAs, whereas WT ezetimibe-treated mice excreted more in the form of NSs. Ezetimibe treatment in Asbt−/− mice caused a further decrease in the total sterol balance.
Fig. 6.
Schematic representation of the total sterol balance over the intestine calculated via dietary cholesterol intake and total sterol output of WT and Asbt−/− mice with and without ezetimibe treatment. Dietary cholesterol intake (A), total fecal sterol excretion (B), and net total sterol balance over the intestine (C). n = 5–6 per group.
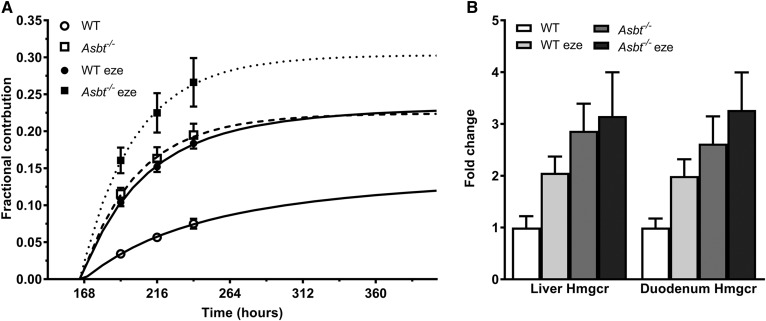
To maintain a steady state in the body, a negative intestinal sterol balance needs to be compensated for by increased cholesterol synthesis. Indeed, enhancing cholesterol disposal via the inhibition of reabsorption induces a compensatory increase in de novo cholesterol synthesis (44). We determined cholesterol synthesis in the four experimental groups using [13C]acetate-labeled drinking water (33). Figure 7A shows the fractional contribution of newly synthesized cholesterol in plasma. Cholesterol synthesis was increased to a similar extent in Asbt−/− and ezetimibe-treated WT mice compared with untreated WT mice. The changes in cholesterol synthesis rates coincided with a similar trend toward a higher expression of Hmgcr in both Asbt−/− groups and WT ezetimibe-treated mice (Fig. 7B). Ezetimibe treatment in Asbt−/− mice further increased cholesterol synthesis compared with Asbt−/− controls (Fig. 7A). The changes in cholesterol absorption and synthesis did not affect plasma cholesterol levels, lipoprotein distribution or hepatic cholesterol levels, or the calculated total body cholesterol pool size (supplemental Figs. S2–S4).
Fig. 7.
Fractional contribution of newly synthesized cholesterol in plasma and changes in cholesterol synthesis upon ezetimibe treatment and Asbt−/− in mice. Hepatic (A) and duodenal (B) Hmgcr gene expression. n = 5–6 per group.
DISCUSSION
We aimed to estimate to what extent cholesterol entering the intestinal lumen via TICE is reabsorbed and thus does not contribute to fecal NS excretion. Using two mechanistically different models of impaired cholesterol (re)absorption, we demonstrate that the net intestinal cholesterol balance is similarly increased. This indicates that efficient reabsorption of TICE strongly limits the disposal of cholesterol from the body under physiological conditions. Therefore, to enhance cholesterol disposal from the body, strategies to stimulate TICE are expected to be most efficacious when they are combined with the simultaneous inhibition of its reabsorption.
Previous studies have shown that ezetimibe increases fecal NS excretion beyond what is expected based upon the decrease in the absorption of dietary and bile-derived cholesterol (18, 19, 22). Up to now, it had not been possible to distinguish conclusively whether the increase in fecal NSs beyond dietary and biliary input was due to the simulation of TICE or to decreased (re)absorption. In the current study, we used a simplified model based on a net intestinal cholesterol balance that was calculated by subtracting dietary and biliary cholesterol input from fecal output (Fig. 2A). In this model, the net intestinal cholesterol balance could be induced via either the stimulation of TICE, reduced (re)absorption, or a combination of both. It should be noted that the net intestinal cholesterol balance encompasses the absorption of cholesterol originating from biliary and dietary origin and the loss of cholesterol in the form of shedding of intestinal cells (at least for the part that is not reabsorbed from the intestine). Based on the provided quantitative calculations and on the estimates in the literature (21, 23), these individual contributions, however, are much smaller than the excretion and reabsorption of transintestinally excreted cholesterol. Our data show that abrogating cholesterol absorption, either directly via ezetimibe or indirectly by reducing the BA pool through Asbt inactivation, elevated the net intestinal cholesterol balance to a similar extent. Under the conditions of impaired cholesterol (re)absorption, the net intestinal cholesterol balance is almost completely determined by TICE. Therefore, we argue that blocking intestinal cholesterol absorption results in increased fecal NS excretion primarily by inhibiting the reabsorption of a basal flux of transintestinally excreted cholesterol into the intestine, which, under physiological conditions, would have been mostly reabsorbed. The reabsorption of transintestinally excreted cholesterol seems to be even more efficient than the (re)absorption of cholesterol from dietary or biliary origin. We cannot exclude that the excretion of cholesterol at the apical membrane of intestinal epithelial cells, that is, close to the site of possible reabsorption, is responsible for this.
The molecular mechanism underlying TICE is not fully understood. One explanation is based on the notion that cholesterol spontaneously transfers from lipoproteins to membranes in various tissues, including the intestine (45). High intestinal bile phospholipid concentrations can potentially be involved in TICE due to their cholesterophilic properties (23, 46). Free cholesterol can transfer to phospholipid-rich intestinal bile content and can subsequently be excreted in the feces. It might be that under physiological conditions there is a basal flux of cholesterol from the blood to the membranes, the intestinal lumen, and back. With either ezetimibe or Asbt deficiency, this basal cholesterol flux is interrupted at the level of reabsorption into the enterocyte. Based on this theory, the slight difference in intestinal cholesterol balance between WT mice treated with ezetimibe versus the inactivation of Asbt (Fig. 2B) could be due to the higher biliary phospholipid secretion in the bile of WT ezetimibe-treated mice compared with Asbt−/− mice (supplemental Fig. S5).
Lumenal BA concentrations and composition and subsequent FXR activation are altered in Asbt−/− mice and might contribute to the observed TICE flux. The exact contribution of intestinal BAs to TICE, especially under physiological conditions, is unclear. BAs are essential for cholesterol absorption through the formation of micelles that travel from the intestinal lumen across the unstirred water layer to the enterocyte. Cholesterol transport in the reverse direction (i.e., from enterocyte to lumen) also requires traveling across the unstirred water layer and could therefore also be (partly) dependent on BAs. However, this might very well occur with a different efficiency. In the original perfusion studies performed by van der Velde et al., hydrophobic BAs (TCA, TDCA) were more efficient in increasing cholesterol in the perfusate (i.e., TICE) compared with hydrophilic species (ursodeoxycholic acid) (23, 24). However, this effect was more dependent on the presence of phospholipids than that of BAs. Moreover, other factors in this artificial system, such as the absence of food or the rate of perfusion, could have affected outcomes and cholesterol (or TICE) reabsorption. In the experiment, supraphysiological doses of BAs were used, and no dose-response curve was established. It therefore remains unclear at what concentration BAs need to be present to cause this increase in TICE. Furthermore, the observations by Van der Velde et al. differ from those by De Boer et al., who showed using bile-diverted rats that the induction of TICE by intestinal FXR activation required a change of biliary BA composition toward a more hydrophilic profile (22). This is in line with another murine study using different FXR agonists and an earlier in vitro observation that ursodeoxycholic acid was more efficient in promoting ABCG5/8-dependent cholesterol efflux than cholic acid (47, 48).
Another explanation for the slight difference in intestinal cholesterol balance could be the different mechanism underlying the reduction of cholesterol absorption in WT ezetimibe-treated mice versus Asbt−/− mice. It is proposed that cholesterol either from the intestinal lumen or from endogenous sources first has to translocate into the brush-border membrane (BBM) before it can be internalized together with NPC1L1 into the enterocyte (30, 49). In Asbt−/− mice the absorption defect is likely due to reduced micelle formation: the decreased intestinal availability of micellar BAs prevents luminal cholesterol from (re-)entering the BBM and thereby its internalization by NPC1L1. Blocking NPC1L1 by ezetimibe is not expected to prevent cholesterol from entering the BBM. Thus, there might be more cholesterol available in the BBM upon ezetimibe treatment compared with the inactivation of Asbt resulting in a higher efflux of cholesterol from the BBM to the lumen from both exo- and endogenous sources (partly mediated via Abcg5/8) (19, 30). This hypothesis is supported by the fact that ezetimibe in Asbt−/− mice did not significantly affect net intestinal cholesterol balance. In Asbt−/− mice cholesterol from the intestine is not expected to reach the BBM, and therefore blocking the internalization with the NPC1L1 process has no relevant additional effect on intestinal and subsequent fecal cholesterol concentrations.
We showed that Asbt−/− mice had a 3-fold increase in fecal BA and NS excretion. While it was previously reported that Asbt−/− mice have decreased fractional cholesterol absorption (55% vs. 74% in WT littermates) (12), it remained unclear whether this could quantitatively account for the strongly increased fecal NS excretion (17, 39). Our data show that fractional cholesterol absorption in Asbt−/− mice was much lower (∼6%) and even similar to that of WT mice treated with ezetimibe (∼4%), suggesting that the increase in fecal NS excretion was mainly due to impaired cholesterol (re)absorption. The differences between the fractional absorption in our experiment and those by Dawson et al. (17) could possibly be explained by differences in the genetic background [C57BL6 in this experiment vs. 129S6/SvEv in Dawson et al. (17)], dietary composition (such as different cholesterol and fiber content), or methodology [dual tracer method with blood sampling in this experiment vs. dual labeled isotope method with fecal sampling in Dawson et al. (17)]. Our conclusion that decreased absorption underlies the increase in fecal NS excretion in Asbt−/− mice was further supported by the finding that treating Asbt−/− mice with ezetimibe did not result in an additional increase in fecal NS excretion. Our interpretation of these two observations is that the increase in fecal NS excretion in Asbt−/− mice is due to decreased intestinal cholesterol (re)absorption.
Although we were able to profoundly increase fecal sterol output via the inactivation of Asbt and/or ezetimibe treatment, plasma cholesterol levels and the total body cholesterol pool did not change (supplemental Figs. S2–S4). Additionally, BW was similar in WT and Asbt−/− mice and unaffected by ezetimibe treatment (data not shown). Maintaining cholesterol homeostasis is essential to the body, as it is an important component of cells and precursor for steroid hormones. It has been shown that mice have a great capacity to maintain the whole-body cholesterol pool size under various conditions (50). However, modulating cholesterol fluxes can affect cholesterol distribution over the different compartments in the body, especially hepatic, plasma, and lipoprotein levels. Ezetimibe has been shown in humans to effectively lower LDL-C while maintaining a constant whole-body cholesterol pool size (51). Current observations together with the previously shown effects on hypercholesterolemia in humans and mice show the profound capacity to modulate cholesterol fluxes and lipoprotein distribution without affecting homeostasis, which is essential for sustaining development. The adaptation of the de novo cholesterol synthesis rate is a major mechanism for balancing the whole-body cholesterol pool size (Fig. 7, supplemental Fig. S4). It can therefore be predicted that simultaneous interference with the cholesterol synthesis capacity will allow more robust manipulations of cholesterol homeostasis, which could contribute to targeted therapeutic strategies.
Challenging our current models by feeding a high-plant sterol or high-cholesterol diet potentially results in more pronounced changes in intestinal cholesterol fluxes. With higher lumenal cholesterol concentrations there could, for example, be a higher membrane cholesterol exchange rate, resulting in a larger difference of the intestinal cholesterol balance between ezetimibe and Asbt inhibition (30). While these experiments are interesting and relevant for the clinical implications of cholesterol absorption inhibiting therapies, it is beyond the scope of our current study, which was specifically aimed at demarcating the role of reabsorption of TICE under physiological conditions.
Ezetimibe did not affect fecal NS excretion in Asbt−/− mice but did augment fecal BA excretion, resulting in increased total fecal sterol excretion. Asbt−/− mice have a profoundly induced Cyp7a1 expression (supplemental Fig. S1), a compensatory reaction to account for fecal BA loss. Therefore, the further augmented cholesterol synthesis by ezetimibe in Asbt−/− mice compared with untreated Asbt−/− mice (Fig. 6A) might be preferentially converted to BAs in the presence of high Cyp7a1 expression. An elevation in hepatic BA synthesis upon ezetimibe treatment in Asbt−/− mice is supported by an increase in fecal BA excretion, which was absent upon ezetimibe treatment in WT mice. Previous studies on the effect of ezetimibe on fecal BA excretion have been conflicting, varying from no change in mice and an increase in humans (19) to no effect in either mice or humans (51). Both ezetimibe and ASBT inhibition have similar benefits on atherosclerosis development in mouse models (52). However, combining both therapies to combat atherosclerosis has to our knowledge not been investigated. While ezetimibe cannot augment NS excretion further in addition to ASBT inhibition, a combination of these two therapeutic strategies might still be beneficial due to the added increase in cholesterol and subsequent BA synthesis.
Altogether, our results demonstrate that most TICE is counteracted by (re)absorption. Based on these findings we propose a model of net intestinal cholesterol balance that represents the resultant flux of intestinal excretion (or TICE) and (re)absorption and is calculated as [fecal NS excretion] − [dietary + biliary cholesterol input] (Fig. 2A). While previous studies have estimated TICE via this calculation, they lacked the concept that TICE is also subjected to reabsorption. Additionally, we showed that the inactivation of Asbt can be used as another model to potently inhibit intestinal cholesterol absorption. Combining the inhibition of intestinal cholesterol (re)absorption with the active induction of TICE is expected to further enhance the disposal of cholesterol from the body and is therefore an interesting target for the prevention and treatment of hypercholesterolemia, with or without additional manipulation of de novo cholesterol synthesis.
Supplementary Material
Acknowledgments
The authors thank Renze Boverhof, Martijn Koehorst, and Rick Havinga for excellent technical assistance and Jan-Freark de Boer, Folkert Kuipers, and Vincent Bloks for their critical comments on the manuscript and valuable suggestions.
Footnotes
Abbreviations:
- ASBT
- apical sodium-dependent bile acid transporter
- BA
- bile acid
- BBM
- brush-border membrane
- BSTFA
- N,O-bis(trimethylsilyl)trifluoroacetamide
- BW
- body weight
- Cyp7a1
- cholesterol 7 α-hydroxylase
- FXR
- farnesoid X receptor
- LDL-C
- LDL-cholesterol
- NPC1L1
- Niemann-Pick C1-like 1
- NS
- neutral sterol
- TCA
- taurocholic acid
- TDCA
- taurodeoxycholic acid
- TICE
- transintestinal cholesterol excretion
- TMCS
- trimethylchlorosilane
- TβMCA
- tauro-β-muricholic acid
This work was supported by The Netherlands Organization for Scientific Research VICI Grant 016.176.640 (J.W.J.) and the European Foundation for the Study of Diabetes (award supported by EFSD/Novo Nordisk). The authors declare no competing financial interests.
The online version of this article (available at http://www.jlr.org) contains a supplement.
REFERENCES
- 1.Gersh B. J., Sliwa K., Mayosi B. M., and Yusuf S.. 2010. The epidemic of cardiovascular disease in the developing world: global implications. Eur. Heart J. 31: 642–648. [DOI] [PubMed] [Google Scholar]
- 2.Lusis A. J. 2000. Atherosclerosis. Nature. 407: 233–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nelson R. H. 2013. Hyperlipidemia as a risk factor for cardiovascular disease. Prim. Care. 40: 195–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hata Y., and Nakajima K.. 2000. Life-style and serum lipids and lipoproteins. J. Atheroscler. Thromb. 7: 177–197. [DOI] [PubMed] [Google Scholar]
- 5.Gepner A. D., Piper M. E., Johnson H. M., Fiore M. C., Baker T. B., and Stein J. H.. 2011. Effects of smoking and smoking cessation on lipids and lipoproteins: outcomes from a randomized clinical trial. Am. Heart J . 161: 145–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ahn C. H., and Choi S. H.. 2015. New drugs for treating dyslipidemia: beyond statins. Diabetes Metab. J. 39: 87–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chaudhary R., Garg J., Shah N., and Sumner A.. 2017. PCSK9 inhibitors: a new era of lipid lowering therapy. World J. Cardiol . 9: 76–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Matsubara T., Li F., and Gonzalez F. J.. 2013. FXR signaling in the enterohepatic system. Mol. Cell. Endocrinol . 368: 17–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cannon C. P., Blazing M. A., Giugliano R. P., McCagg A., White J. A., Theroux P., Darius H., Lewis B. S., Ophuis T. O., Jukema J. W., et al. . 2015. Ezetimibe added to statin therapy after acute coronary syndromes. N. Engl. J. Med . 372: 2387–2397. [DOI] [PubMed] [Google Scholar]
- 10.Einarsson K., Ericsson S., Ewerth S., Reihnér E., Rudling M., Stahlberg D., and Angelin B.. 1991. Bile acid sequestrants: mechanisms of action on bile acid and cholesterol metabolism. Eur. J. Clin. Pharmacol. 40: S53–S58. [PubMed] [Google Scholar]
- 11.Mazidi M., Rezaie P., Karimi E., and Kengne A. P.. 2017. The effects of bile acid sequestrants on lipid profile and blood glucose concentrations: a systematic review and meta-analysis of randomized controlled trials. Int. J. Cardiol . 227: 850–857. [DOI] [PubMed] [Google Scholar]
- 12.Lan T., Haywood J., and Dawson P. A.. 2013. Inhibition of ileal apical but not basolateral bile acid transport reduces atherosclerosis in apoE−/− mice. Atherosclerosis. 229: 374–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bhat B. G. 2003. Inhibition of ileal bile acid transport and reduced atherosclerosis in apoE−/− mice by SC-435. J. Lipid Res. 44: 1614–1621. [DOI] [PubMed] [Google Scholar]
- 14.Kitayama K., Nakai D., Kono K., van der Hoop A. G., Kurata H., de Wit E. C., Cohen L. H., Inaba T., and Kohama T.. 2006. Novel non-systemic inhibitor of ileal apical Na+-dependent bile acid transporter reduces serum cholesterol levels in hamsters and monkeys. Eur. J. Pharmacol. 539: 89–98. [DOI] [PubMed] [Google Scholar]
- 15.West K. L., Zern T. L., Butteiger D. N., Keller B. T., and Fernandez M. L.. 2003. SC-435, an ileal apical sodium co-dependent bile acid transporter (ASBT) inhibitor lowers plasma cholesterol and reduces atherosclerosis in guinea pigs. Atherosclerosis. 171: 201–210. [DOI] [PubMed] [Google Scholar]
- 16.Dawson P. A. 2017. Roles of ileal ASBT and OSTα-OSTβ in regulating bile acid signaling. Dig. Dis. 35: 261–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dawson P. A., Haywood J., Craddock A. L., Wilson M., Tietjen M., Kluckman K., Maeda N., and Parks J. S.. 2003. Targeted deletion of the ileal bile acid transporter eliminates enterohepatic cycling of bile acids in mice. J. Biol. Chem. 278: 33920–33927. [DOI] [PubMed] [Google Scholar]
- 18.Vrins C. L. J. 2010. From blood to gut: direct secretion of cholesterol via transintestinal cholesterol efflux. World J. Gastroenterol. 16: 5953–5957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jakulj L., van Dijk T. H., Freark de Boer J., Kootte R. S., Schonewille M., Paalvast Y., Boer T., Bloks V. W., Boverhof R., Nieuwdorp M., et al. . 2016. Transintestinal cholesterol transport is active in mice and humans and controls ezetimibe-induced fecal neutral sterol excretion. Cell Metab . 24: 783–794. [DOI] [PubMed] [Google Scholar]
- 20.Brufau G., Kuipers F., Lin Y., Trautwein E. A., and Groen A. K.. 2011. A reappraisal of the mechanism by which plant sterols promote neutral sterol loss in mice. PLoS One. 6: e21576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.van der Veen J. N., van Dijk T. H., Vrins C. L. J., van Meer H., Havinga R., Bijsterveld K., Tietge U. J. F., Groen A. K., and Kuipers F.. 2009. Activation of the liver X receptor stimulates trans-intestinal excretion of plasma cholesterol. J. Biol. Chem. 284: 19211–19219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.de Boer J. F., Schonewille M., Boesjes M., Wolters H., Bloks V. W., Bos T., van Dijk T. H., Jurdzinski A., Boverhof R., Wolters J. C., et al. . 2017. Intestinal farnesoid X receptor controls transintestinal cholesterol excretion in mice. Gastroenterology. 152: 1126–1138.e6. [DOI] [PubMed] [Google Scholar]
- 23.van der Velde A. E., Vrins C. L. J., van den Oever K., Kunne C., Oude Elferink R. P. J., Kuipers F., and Groen A. K.. 2007. Direct intestinal cholesterol secretion contributes significantly to total fecal neutral sterol excretion in mice. Gastroenterology. 133: 967–975. [DOI] [PubMed] [Google Scholar]
- 24.van der Velde A. E., Vrins C. L. J., van den Oever K., Seemann I., Oude Elferink R. P. J., van Eck M., Kuipers F., and Groen A. K.. 2008. Regulation of direct transintestinal cholesterol excretion in mice. Am. J. Physiol. Liver Physiol . 295: G203–G208. [DOI] [PubMed] [Google Scholar]
- 25.de Boer J. F., Kuipers F., and Groen A. K.. 2018. Cholesterol transport revisited: a new turbo mechanism to drive cholesterol excretion. Trends Endocrinol. Metab . 29: 123–133. [DOI] [PubMed] [Google Scholar]
- 26.Reeskamp L. F., Meessen E. C. E., and Groen A. K.. 2018. Transintestinal cholesterol excretion in humans. Curr. Opin. Lipidol. 29: 10–17. [DOI] [PubMed] [Google Scholar]
- 27.Kruit J. K., Plösch T., Havinga R., Boverhof R., Groot P. H. E., Groen A. K., and Kuipers F.. 2005. Increased fecal neutral sterol loss upon liver X receptor activation is independent of biliary sterol secretion in mice. Gastroenterology. 128: 147–156. [DOI] [PubMed] [Google Scholar]
- 28.Voshol P. J., Havinga R., Wolters H., Ottenhoff R., Princen H. M. G., Elferink R. P. J. O., Groen A. K., and Kuipers F.. 1998. Reduced plasma cholesterol and increased fecal sterol loss in multidrug resistance gene 2 P-glycoprotein-deficient mice. Gastroenterology. 114: 1024–1034. [DOI] [PubMed] [Google Scholar]
- 29.Jakulj L., Vissers M. N., van Roomen C. P., van der Veen J. N., Vrins C. L. J., Kunne C., Stellaard F., Kastelein J. J. P., and Groen A. K.. 2010. Ezetimibe stimulates faecal neutral sterol excretion depending on abcg8 function in mice. FEBS Lett . 584: 3625–3628. [DOI] [PubMed] [Google Scholar]
- 30.Nakano T., Inoue I., Takenaka Y., Ono H., Katayama S., Awata T., and Murakoshi T.. 2016. Ezetimibe promotes brush border membrane-to-lumen cholesterol efflux in the small intestine. PLoS One. 11: e0152207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Turley S. D., Schwarz M., Spady D. K., and Dietschy J. M.. 1998. Gender-related differences in bile acid and sterol metabolism in outbred CD-1 mice fed low- and high-cholesterol diets. Hepatology. 28: 1088–1094. [DOI] [PubMed] [Google Scholar]
- 32.Schonewille M., Freark de Boer J., Mele L., Wolters H., Bloks V. W., Wolters J. C., Kuivenhoven J. A., Tietge U. J. F., Brufau G., and Groen A. K.. 2016. Statins increase hepatic cholesterol synthesis and stimulate fecal cholesterol elimination in mice. J. Lipid Res. 57: 1455–1464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ronda O. A. H. O., van Dijk T. H., Verkade H. J., and Groen A. K.. 2016. Measurement of intestinal and peripheral cholesterol fluxes by a dual-tracer balance method. Curr. Protoc. Mouse Biol. 6: 408–434. [DOI] [PubMed] [Google Scholar]
- 34.Kuipers F., van Ree J. M., Hofker M. H., Wolters H., In’t Veld G., Havinga R., Vonk R. J., Princen H. M., and Havekes L. M.. 1996. Altered lipid metabolism in apolipoprotein E-deficient mice does not affect cholesterol balance across the liver. Hepatology. 24: 241–247. [DOI] [PubMed] [Google Scholar]
- 35.Out C., Patankar J. V., Doktorova M., Boesjes M., Bos T., De Boer S., Havinga R., Wolters H., Boverhof R., Van Dijk T. H., Smoczek A., Bleich A., Sachdev V., Kratky D., Kuipers F., Verkade H. J., and Groen A. K.. 2015. Gut microbiota inhibit Asbt-dependent intestinal bile acid reabsorption via Gata4. J. Hepatol. 63: 697–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bligh E. G., and Dyer W. J.. 1959. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 37: 911–917. [DOI] [PubMed] [Google Scholar]
- 37.Ge L., Wang J., Qi W., Miao H. H., Cao J., Qu Y. X., Li B. L., and Song B. L.. 2008. The cholesterol absorption inhibitor ezetimibe acts by blocking the sterol-induced internalization of NPC1L1. Cell Metab. 7: 508–519. [DOI] [PubMed] [Google Scholar]
- 38.Altmann S. W., Davis H. R., Zhu L.-J., Yao X., Hoos L. M., Tetzloff G., Iyer S. P. N., Maguire M., Golovko A., Zeng M., Wang L., Murgolo N., and Graziano M. P.. 2004. Niemann-Pick C1 like 1 protein is critical for intestinal cholesterol absorption. Science. 303: 1201–1204. [DOI] [PubMed] [Google Scholar]
- 39.Dawson P. A. 2015. Impact of inhibiting ileal apical versus basolateral bile acid transport on cholesterol metabolism and atherosclerosis in mice. Dig. Dis. 33: 382–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang D. Q-H., Tazuma S., Cohen D. E., and Carey M. C.. 2003. Feeding natural hydrophilic bile acids inhibits intestinal cholesterol absorption: studies in the gallstone-susceptible mouse. Am. J. Physiol. Gastrointest. Liver Physiol. 285: G494–G502. [DOI] [PubMed] [Google Scholar]
- 41.Heuman D. M. 1989. Quantitative estimation of the hydrophilic-hydrophobic balance of mixed bile salt solutions. J. Lipid Res. 30: 719–730. [PubMed] [Google Scholar]
- 42.Gallo-Torres H. E., Miller O. N., and Hamilton J. G.. 1971. Further studies on the role of bile salts in cholesterol esterification and absorption from the gut. Arch. Biochem. Biophys. 143: 22–36. [DOI] [PubMed] [Google Scholar]
- 43.Zhao C., and Dahlman-Wright K.. 2010. Liver X receptor in cholesterol metabolism. J. Endocrinol. 204: 233–240. [DOI] [PubMed] [Google Scholar]
- 44.Sudhop T., Lutjohann D., Kodal A., Tribble D. L., Shah S., Perevozskaya I., and Von Bergmann K.. 2002. Inhibition of intestinal cholesterol absorption by ezetimibe in humans. Circulation. 106: 1943–1948. [DOI] [PubMed] [Google Scholar]
- 45.Gillard B. K., Rosales C., Xu B., Gotto A. M. Jr., and Pownall H. J.. 2018. Rethinking reverse cholesterol transport and dysfunctional high-density lipoproteins. J. Clin. Lipidol . 12: 849–856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Niu S. L., and Litman B. J.. 2002. Determination of membrane cholesterol partition coefficient using a lipid vesicle-cyclodextrin binary system: effect of phospholipid acyl chain unsaturation and headgroup composition. Biophys. J. 83: 3408–3415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Vrins C., Vink E., Vandenberghe K. E., Frijters R., Seppen J., and Groen A. K.. 2007. The sterol transporting heterodimer ABCG5/ABCG8 requires bile salts to mediate cholesterol efflux. FEBS Lett. 581: 4616–4620. [DOI] [PubMed] [Google Scholar]
- 48.Xu Y., Li F., Zalzala M., Xu J., Gonzalez F. J., Adorini L., Lee Y., Yin L., and Zhang Y.. 2016. Farnesoid X receptor activation increases reverse cholesterol transport by modulating bile acid composition and cholesterol absorption in mice. Hepatology. 64: 1072–1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nakano T., Inoue I., and Murakoshi T.. 2019. A newly integrated model for intestinal cholesterol absorption and efflux reappraises how plant sterol intake reduces circulating cholesterol levels. Nutrients. 11: E310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dietschy J. M., and Turley S. D.. 2002. Control of cholesterol turnover in the mouse. J. Biol. Chem. 277: 3801–3804. [DOI] [PubMed] [Google Scholar]
- 51.Lin X., Racette S. B., Ma L., Wallendorf M., and Ostlund R. E.. 2017. Ezetimibe increases endogenous cholesterol excretion in humans. Arterioscler. Thromb. Vasc. Biol. 37: 990–996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Braun A., Yesilaltay A., Acton S., Broschat K. O., Krul E. S., Napawan N., Stagliano N., and Krieger M.. 2008. Inhibition of intestinal absorption of cholesterol by ezetimibe or bile acids by SC-435 alters lipoprotein metabolism and extends the lifespan of SR-BI/apoE double knockout mice. Atherosclerosis. 198: 77–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.