Abstract
The ubiquitous chlorophyll a (Chl a) pigment absorbs both blue and red light. Yet, in contrast to green algae and higher plants, most cyanobacteria have much lower photosynthetic rates in blue than in red light. A plausible but not yet well-supported hypothesis is that blue light results in limited energy transfer to photosystem II (PSII), because cyanobacteria invest most Chl a in photosystem I (PSI), whereas their phycobilisomes (PBS) are mostly associated with PSII but do not absorb blue photons. In this paper, we compare the photosynthetic performance in blue and orange-red light of wildtype Synechocystis sp. PCC 6803 and a PBS-deficient mutant. Our results show that the wildtype had much lower biomass, Chl a content, PSI:PSII ratio and O2 production rate per PSII in blue light than in orange-red light, whereas the PBS-deficient mutant had a low biomass, Chl a content, PSI:PSII ratio, and O2 production rate per PSII in both light colors. More specifically, the wildtype displayed a similar low photosynthetic efficiency in blue light as the PBS-deficient mutant in both light colors. Our results demonstrate that the absorption of light energy by PBS and subsequent transfer to PSII are crucial for efficient photosynthesis in cyanobacteria, which may explain both the low photosynthetic efficiency of PBS-containing cyanobacteria and the evolutionary success of chlorophyll-based light-harvesting antennae in environments dominated by blue light.
Keywords: Blue light, Photosynthesis, Phycobilisomes, Synechocystis sp. PCC 6803, PAL mutant, Oxygen production
Introduction
Cyanobacteria are the oldest known oxygen-producing photosynthetic organisms on our planet and their photosynthetic activity is widely held responsible for oxygenation of the Earth’s atmosphere about 2.3 billion years ago (Holland 2002; Schirrmeister et al. 2015). The ubiquitous chlorophyll a (Chl a) pigment in the photosystems of oxygenic phototrophs absorbs both blue (440 nm) and red light (678 nm), and hence one would expect that these two colors of light are used at approximately equal efficiency. Yet, in contrast to green algae and higher plants, cyanobacteria appear to have much lower oxygen (O2) production and growth rates in blue light than in red light (e.g., Lemasson et al. 1973; Pulich and van Baalen 1974; Wilde et al. 1997; Tyystjärvi et al. 2002; Wang et al. 2007; Singh et al. 2009; Luimstra et al. 2018).
Why cyanobacteria perform less well in blue light is not yet fully resolved. It is often hypothesized that the distribution of absorbed light energy between photosystem I (PSI) and photosystem II (PSII) plays a key role (Fujita 1997; El Bissati and Kirilovsky 2001; Wang et al. 2007; Singh et al. 2009; Solhaug et al. 2014; Kirilovsky 2015; Luimstra et al. 2018). To optimize absorption of light energy, cyanobacteria use phycobilisomes (PBS) as light-harvesting antennae, which transfer absorbed light energy to the photosystems (Grossman et al. 1993; Tandeau de Marsac 2003). Most of the PBS are typically associated with PSII (state 1), but PBS can be relocated to PSI (state 2) at time scales of seconds to minutes, or they can be detached from the photosystems in which case they do not contribute to the photosynthetic activity of the cells (Joshua et al. 2005; Mullineaux 2014; Kirilovsky 2015). Blue light ≤ 450 nm is very poorly absorbed by PBS (Tandeau de Marsac 2003; Six et al. 2007), however, and hence PBS cannot distribute the absorbed light energy over the two photosystems.
Instead, blue light is directly absorbed by chlorophyll and carotenoids associated with the two photosystems. Cyanobacteria contain ~ 100 molecules of Chl a per PSI (Jordan et al. 2001) but only ~ 35 molecules of Chl a per PSII (Umena et al. 2011). Moreover, the PSI:PSII ratio of cyanobacteria usually ranges between 5:1 and 2:1, depending on the growth conditions, which is higher than the approximately 1:1 ratio often found in eukaryotic phototrophs (Shen et al. 1993; Fujita 1997; Olive et al. 1997; Singh et al. 2009; Allahverdiyeva et al. 2014). Since cyanobacteria usually invest most of their Chl a in PSI (e.g., Myers et al. 1980; Fujita 1997; Luimstra et al. 2018) and, in cyanobacteria, only the carotenoids in PSI are involved in light harvesting (Stamatakis et al. 2014), PSI will absorb more blue photons than PSII. This implies that blue light is likely to cause an excitation imbalance between both photosystems, with low O2 production by PSII and limited linear electron transport (Fujita 1997; Solhaug et al. 2014; Kirilovsky 2015), which then explains the low photosynthetic efficiency and growth rate of cyanobacteria in blue light (Luimstra et al. 2018). However, although many observations support this hypothesis, conclusive evidence is still limited.
In this paper, we aim to contribute to a further understanding of why cyanobacteria display a low photosynthetic efficiency in blue light. Therefore, we compare the photosynthetic efficiency in blue and orange-red light of Synechocystis sp. PCC 6803 and a mutant strain lacking PBS known as the PAL mutant (Ajlani and Vernotte 1998). The PAL mutant does not have light-harvesting antennae that are able to redistribute light energy over both photosystems. Consequently, our expectation is that the PAL mutant will have limited energy transfer to PSII irrespective of the light color and hence will display a similarly low photosynthetic efficiency in both orange-red and blue light as the wildtype in blue light. An advanced understanding of the light-color dependence of cyanobacterial photosynthesis may contribute to an improved design of successful culture conditions in biotechnological applications, and to further clarification of the ecological distributions of cyanobacteria in waters dominated by different wavelengths.
Materials and methods
Strains and culture conditions
We investigated a wildtype and a mutant strain of Synechocystis sp. PCC 6803, which uses the phycobilin-pigment C-phycocyanin in its PBS. The wildtype strain was provided by professor D. Bhaya (University of Stanford, USA) and the phycobilisome-deficient PAL mutant strain was provided by Dr. G. Ajlani (Université Paris-Sud, France).
The experimental set-up is illustrated in Fig. 1a,b. The strains were grown at 30 °C in 1.8 L light-limited chemostats with flat culture vessels illuminated from one side (Huisman et al. 2002). Blue light (450 nm) or orange-red light (660 nm) with a full width at half maximum of ~ 20 nm was provided by narrow-band LED panels (Philips Lighting B.V., Eindhoven, The Netherlands) at an incident light intensity of 45 µmol photons m−2 s−1. Light intensities were measured with an LI-250 light meter (LI-COR Biosciences, Lincoln, NE, USA). The chemostats had an optical path length of 5 cm. Cultures were provided with BG-11 medium (Merck, Darmstadt, Germany) supplemented with 5 mM Na2CO3, at a constant dilution rate of D = 0.015 h−1 (0.36 day−1). Chloramphenicol (20 µg mL−1) was added to the first 6 L of medium for the PAL mutant to prevent growth of the wildtype (Ajlani and Vernotte 1998). The cultures were mixed by bubbling with CO2-enriched air (2% v/v) flowing at a rate of 30 L h−1. The CO2 concentration of the gas mixture was regularly monitored using an Environmental Gas Monitor for CO2 (EGM-4; PP Systems, Amesbury, MA, USA).
Fig. 1.
Synechocystis sp. PCC 6803 wildtype and the PAL mutant were grown in light-limited chemostats and provided with either a orange-red (660 nm) or b blue (450 nm) LED light. Insets show samples taken from steady-state chemostats of the wildtype, which illustrate that the wildtype produced much higher biomass in orange-red than in blue light. Bar graphs show biomass (c), cell counts (d) and cellular Chl a content (e) of each strain at steady state in orange-red (R) and blue (B) light. Biomass is expressed as total biovolume of the cells per L. Data show the averages of three (biomass and cell counts) or two (Chl a content) technical replicates ± SD
Cell counts and chlorophyll analysis
A CASY 1 TTC cell counter with a 60-µm capillary (Schärfe Systems GmbH, Reutlingen, Germany) was used to count cells and measure their biovolume. Samples were diluted to ~ 5×104 cells mL−1 in Casyton solution prior to measuring. Chl a content of the cells was measured spectrophotometrically using a Novaspec III Visible Spectrophotometer after extraction in 80% (v/v) acetone/5% (v/v) DMSO (Porra et al. 1989).
Absorption and 77 K fluorescence spectra
After the chemostats reached steady state, 1 mL and 1.5 mL samples were taken for the measurement of light absorption spectra and 77 K fluorescence spectra, respectively. Immediately after sampling, light absorption spectra were measured from 400 to 750 nm using an updated Aminco DW2000 spectrophotometer (OLIS, Bogart, GA, USA). The spectra were normalized to Chl a absorbance at 678 nm, after baseline correction for minimum absorbance at 750 nm. Molar phycocyanin-to-Chl a (PC:Chl) ratios were estimated from the absorption spectra according to Rakhimberdieva et al. (2001):
where A625, A652, and A678 are the absorption peaks of PC, allophycocyanin, and Chl a, respectively. Cellular PC contents (quantified as monomers) were calculated from the measured Chl a content and estimated PC:Chl ratio.
For the 77 K fluorescence spectra, the 1.5 mL samples were transferred to 3-mL cuvettes prefilled with 1.5 mL 60% glycerol. The final glycerol concentration in the cuvettes was 30% to minimize dissociation of PBS, and cell concentrations were below 4 × 107 cells mL−1 to minimize re-absorption of the fluorescence signal (Mao et al. 2003). Samples were mixed by pipetting up and down three times and the cuvettes were frozen in liquid nitrogen. To guarantee that the fluorescence spectra reflected the quantities of photosynthetic components at the time of sampling, this procedure was performed within 20 s. Cuvettes were stored at − 80 °C, for maximally 1 week, until 77 K fluorescence analysis with an OLIS DM45 spectrofluorimeter (OLIS, Bogart, GA, USA) equipped with a Dewar cell. Fluorescence emission was measured from 630 to 750 nm with excitation at 440 nm (mainly Chl a). The peak areas of PSI (emission at 725 nm) and PSII (emission at 695 nm) were calculated by deconvolution of the spectra according to Du et al. (2016) using R version 3.3.3 (R Development Core Team 2017).
Earlier studies have shown that the ratio of the surface areas of the PSI and PSII peaks in the 77 K fluorescence emission spectra corresponds quite well to the molar PSI:PSII ratio of the actual protein complexes (Murakami 1997; Schuurmans et al. 2017). Therefore, cellular PSI and PSII contents (in mol cell−1) were calculated using the PSI:PSII ratio obtained from the deconvoluted 77 K spectra and the cellular Chl a content (mol cell−1, where Chl a has a molecular weight of 893.5 g mol−1). The calculation assumes that PSI and PSII contain 100 and 35 Chl a molecules, respectively (Jordan et al. 2001; Umena et al. 2011):
We note that these calculations should be interpreted with care, and provide only a rough estimate of relative changes in PSI and PSII content.
Oxygen production and consumption rates analyzed using MIMS
O2 production and consumption were measured using membrane-inlet mass spectrometry (MIMS), via high-vacuum-supported diffusive equilibration of the dissolved O2 through a gas permeable membrane coupled to a mass spectrometer (Kana et al. 1994). Small amounts of gasses can flow out of the liquid culture into the sensor of the mass spectrometer through a thin Teflon membrane. Addition of 18O2 and subsequent quantification of both stable oxygen isotopes (16O2 and 18O2) allows for distinction between O2 production (evolution of 16O2 from water-splitting at PSII) and O2 consumption (uptake of the added 18O2) in a single sample. Samples were taken from the steady-state continuous cultures of Synechocystis sp. PCC 6803 wildtype and the PAL mutant acclimated to orange-red and blue light. To facilitate comparison, all samples were diluted to the same OD750 of 0.04 prior to the MIMS analysis.
A fresh sample was taken for each measurement and transferred to a double-walled, airtight 10-mL glass chamber (a DW3 cuvette from Hansatech Instruments, modified by the Technology Centre of the University of Amsterdam) equipped with a magnetic stirrer. The inlet sensor of the mass spectrometer was placed directly in the culture. Prior to measurements, NaHCO3 was added at a final concentration of 15 mM to prevent carbon limitation during the experiment. The samples were flushed with N2 gas to reduce the 16O2 concentration to ~ 5% of the value in air-equilibrated medium to prevent O2 saturation during the experiment, after which 18O2 (95–98% pure; Cambridge Isotope Laboratories) was added in the headspace. The magnetic stirrer allowed for diffusion into the liquid culture. When the 18O2 concentration reached ~ 20% of the value in air-equilibrated medium, the headspace was aspirated and the glass chamber closed.
Temperature in the glass chamber was maintained at 30 °C by water pumped through its double wall. Two LED lamps, identical to those used for the chemostats, illuminated the glass chamber from both sides to minimize shading effects. The samples were exposed to orange-red or blue light at increasing light intensities (summed over both lamps) from 0 to 400 µmol photons m−2 s−1. After 10-min dark adaptation, light intensity was increased every 3 min, while O2 concentrations were measured continuously by the MIMS. The MIMS signals were normalized to argon according to Kana et al. (1994), and O2 production and consumption rates were calculated according to Bañares-España et al. (2013).
O2 production and consumption rates were expressed as fmol O2 per cell per minute and as mol O2 per mol PSII per second. Cell densities were determined directly after each MIMS experiment. Gross O2 production (P) rates were plotted versus light intensity (I) and fitted to P–I curves using a hyperbolic tangent function (Platt and Jassby 1976):
where Pmax is the maximal rate of O2 production and α is the affinity for light (i.e., the initial slope of the P–I curve). The fits were based on a nonlinear least-squares regression using R version 3.3.3 (R Development Core Team 2017).
Results
The PAL mutant produced less biomass in both orange-red and blue light
Photos of the steady-state chemostats illustrate the conspicuous difference in growth performance between Synechocystis sp. PCC 6803 wildtype grown in orange-red (Fig. 1a) and in blue light (Fig. 1b). Both strains grew sufficiently well to sustain a steady state, which implies that their maximum specific growth rates exceeded the dilution rate of 0.015 h−1 in both light colors. However, the wildtype produced much higher steady-state biomass and cell numbers in orange-red light than in blue light (Fig. 1c, d). By contrast, the steady-state biomass and cell numbers of the PAL mutant were low in both orange-red and blue light, and comparable to those of the wildtype in blue light (Fig. 1c, d).
Furthermore, the wildtype had a higher Chl a content per cell in orange-red light than in blue light, whereas the PAL mutant had a low Chl a content in both orange-red and blue light (Fig. 1e). The wildtype had a lower cell volume in orange-red light (5.4 ± 0.03 µm3) than in blue light (7.4 ± 0.03 µm3), whereas the PAL mutant produced smaller cells in both light colors (3.6 ± 0.1 µm3).
The PAL mutant showed identical absorption spectra in both orange-red and blue light
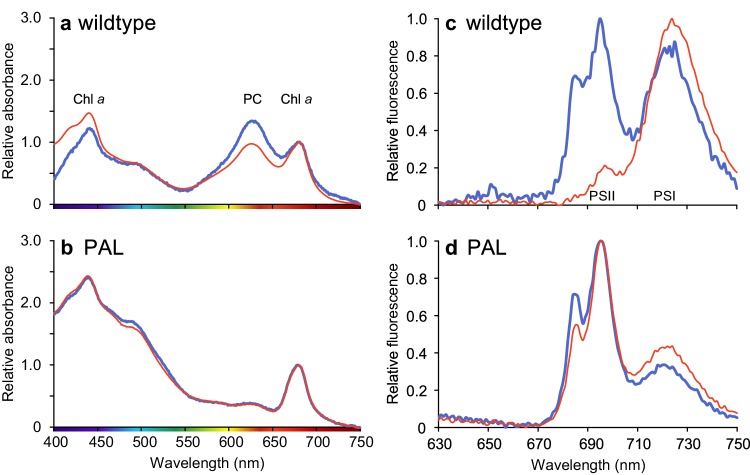
Absorption spectra normalized to Chl a revealed that Synechocystis sp. PCC 6803 wildtype had lower PC:Chl ratios in orange-red light than in blue light (Fig. 2a; Table 1). By contrast, the PAL mutant lacks absorption by PC (at 625 nm) and its absorption spectra were completely identical when grown in orange-red and blue light (Fig. 2b). Furthermore, the PAL mutant had a bleached appearance with a low Chl a content per cell and showed higher absorption in the blue part of the spectrum, both when acclimated to blue and when acclimated to orange-red light (Fig. 2b).
Fig. 2.
(a, b) Light absorption spectra of Synechocystis sp. PCC 6803 wildtype (a) and PAL mutant (b) sampled from steady-state chemostats acclimated to either orange-red (red line) or blue (blue line) light. Main absorption peaks of chlorophyll a (Chl a) and phycocyanin (PC) are indicated in panel a. Light absorption was normalized to Chl a absorbance at 678 nm, after baseline correction for minimum absorbance at 750 nm. c, d Low-temperature (77 K) fluorescence emission spectra of Synechocystis sp. PCC 6803 wildtype (c) and PAL mutant (d). Excitation of the cells at 440 nm (mainly Chl a) yields fluorescence emission peaks around 695 nm for PSII and around 720 nm for PSI, as indicated in panel c. The 77 K fluorescence emission spectra were normalized to the minimum and maximum emission of each spectrum. All spectra show the averages of three technical replicates
Table 1.
Cellular contents of Chl a, PC, PSI and PSII of the wildtype and PAL mutant, in steady-state chemostats acclimated to either orange-red or blue light
| Wildtype | PAL mutant | Units | |||
|---|---|---|---|---|---|
| Orange-red | Blue | Orange-red | Blue | ||
| Chl a contenta | 45 | 28 | 15 | 14 | 10−18 mol cell−1 |
| PSI contentb | 0.43 | 0.22 | 0.11 | 0.09 | 10−18 mol cell−1 |
| PSII contentb | 0.04 | 0.15 | 0.12 | 0.14 | 10−18 mol cell−1 |
| PSI:PSII ratioc | 11.7 | 1.5 | 0.9 | 0.7 | – |
| PC contentd | 8.0 | 7.4 | n.a. | n.a. | 10−18 mol cell−1 |
| PC:Chl ratioe | 0.18 | 0.26 | n.a. | n.a. | – |
| PC:PSII ratiof | 217 | 50 | n.a. | n.a. | – |
aChl a content was measured spectrophotometrically
bPSI and PSII content were calculated from the Chl a content and PSI:PSII ratio
cPSI:PSII ratio was estimated from deconvolution of the 77 K fluorescence spectra
dPC content was calculated from the Chl a content and PC:Chl ratio
ePC:Chl ratio was estimated from the absorption spectra
fPC:PSII ratio was calculated from the PC and PSII content
The PAL mutant had a low PSI:PSII ratio in both orange-red and blue light
Fluorescence peaks of PSI and PSII at 725 nm and 695 nm, respectively, were very prominent in the 77 K fluorescence spectra (Fig. 2c, d). The wildtype allocated most of its Chl a to PSI when grown in orange-red light, whereas it decreased its allocation to PSI and increased its allocation to PSII when grown in blue light (Fig. 2c). Hence, the wildtype had much lower PSI:PSII ratios and PC:PSII ratios in blue light than in orange-red light (Table 1). By contrast, the PAL mutant had a relatively low PSI content and high PSII content in both light colors, resulting in a low PSI:PSII ratio in both orange-red and blue light (Fig. 2d; Table 1).
The blue-light acclimated wildtype showed an additional fluorescence peak at 685 nm (Fig. 2c), which is commonly attributed to the chlorophyll antenna CP43 in PSII (Andrizhiyevskaya et al. 2005; Wilson et al. 2007; Brecht et al. 2014). In the PAL mutant, this peak was present in both orange-red and blue light (Fig. 2d).
The PAL mutant had a low oxygen production in both orange-red and blue light
Membrane-inlet mass spectrometry (MIMS) was used to separately measure photosynthetic O2 production and O2 consumption at different light intensities. O2 production and consumption rates were expressed in two different ways: per mol PSII (Fig. 3) and per cell (Fig. 4). We note that our estimates of the O2 production rate per PSII are consistent with turnover rates of the PSII-water oxidizing complex measured in microalgae (Ananyev and Dismukes 2005).
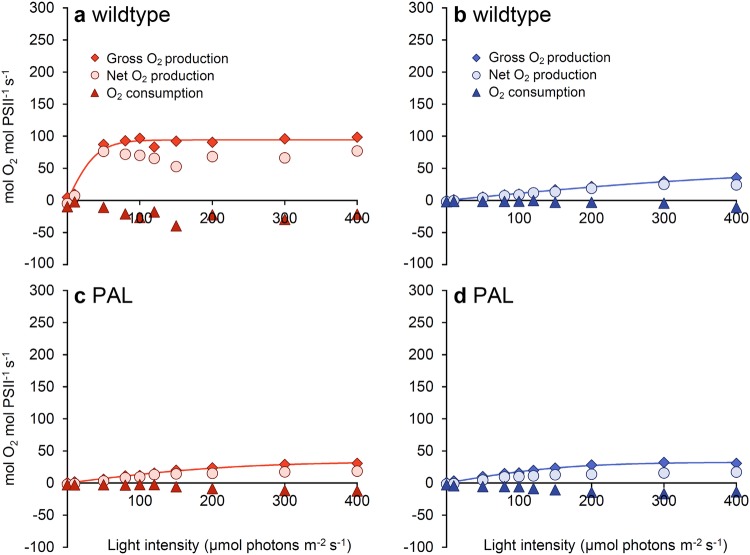
Fig. 3.
O2 production and consumption rates per PSII of Synechocystis sp. PCC 6803 wildtype (a, b) and the PAL mutant (c, d). Cells were acclimated to orange-red (a, c) or blue (b, d) light and subsequently exposed to increasing intensities of the same light color. The data show averages of gross photosynthetic O2 production (diamonds), net O2 production (circles), and O2 consumption (triangles), measured in duplicate samples using membrane-inlet mass spectrometry (MIMS). Lines represent P–I curves fitted to the gross O2 production using the hyperbolic tangent function; parameter estimates are given in Table 2
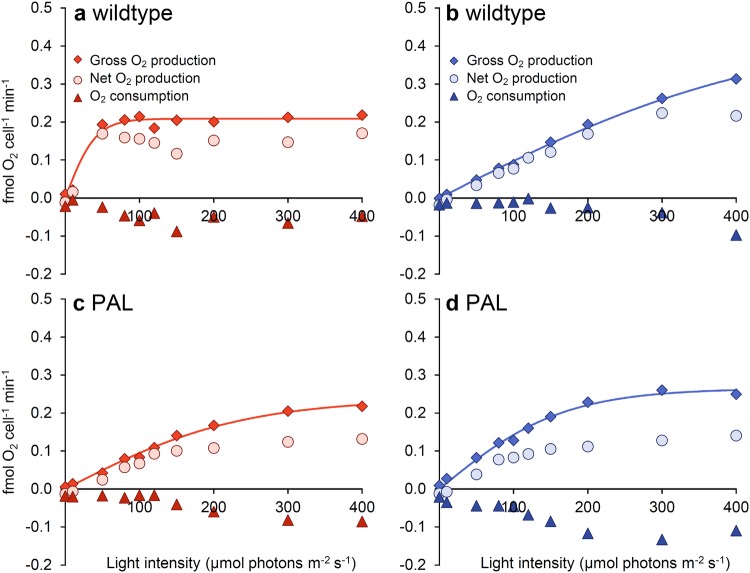
Fig. 4.
O2 production and consumption rates per cell of Synechocystis sp. PCC 6803 wildtype (a, b) and the PAL mutant (c, d). See Fig. 3 for further details
In the wildtype, net and gross O2 production per PSII increased much more steeply with light intensity (had higher α) and maximum O2 production rates per PSII (higher Pmax) were higher in orange-red than in blue light (Fig. 3a, b; Table 2). By contrast, the PAL mutant had low α and low Pmax in both orange-red and blue light (Fig. 3c, d), very similar to the low α and low Pmax of the wildtype in blue light (Fig. 3b).
Table 2.
Photosynthetic parameters estimated by fitting a hyperbolic tangent to the gross O2 production rate per PSII and the gross O2 production rate per cell. Standard errors are indicated by bracketed values
| Parameter | Wildtype | PAL mutant | ||
|---|---|---|---|---|
| Orange-red | Blue | Orange-red | Blue | |
| O2 production per PSII (mol O2 mol PSII−1 s−1) | ||||
| α | 2.32 (0.43) | 0.11 (0.00) | 0.14 (0.01) | 0.20 (0.01) |
| Pmax | 94.2 (3.1) | 50.1 (3.5) | 33.6 (1.3) | 32.6 (1.0) |
| O2 production per cell (fmol O2 cell−1 min−1) | ||||
| α | 5.1 × 10−3 (9.6 × 10−4) | 1.0 × 10−3 (0.3 × 10−4) | 1.0 × 10−3 (0.4 × 10−4) | 1.6 × 10−3 (0.7 × 10−4) |
| Pmax | 0.209 (0.007) | 0.446 (0.032) | 0.237 (0.009) | 0.265 (0.008) |
When O2 production and consumption rates were expressed per cell rather than per PSII, we obtained similar results (Fig. 4). The wildtype again had higher α in orange-red light than in blue light (Fig. 4a, b; Table 2), whereas the PAL mutant had a low α in both orange-red and blue light comparable to the low α of the other strains in blue light (Fig. 4c, d). However, in contrast to Pmax per PSII, Pmax per cell was of similar magnitude in blue and orange-red light for both strains (Fig. 4).
Discussion
What happens if phycobilisomes cannot be used?
Our results show that Synechocystis sp. PCC 6803 wildtype displays much lower O2 and biomass production rates in blue light than in orange-red light, in agreement with previous studies (e.g., Wilde et al. 1997; El Bissati and Kirilovsky 2001; Tyystjärvi et al. 2002; Singh et al. 2009; Bland and Angenent 2016; Luimstra et al. 2018). We tested the hypothesis that cyanobacteria have a low photosynthetic efficiency in blue light because PBS do not absorb wavelengths ≤ 450 nm, and hence blue light mainly excites PSI while fewer photons excite the PSII reaction center (Myers et al. 1980; Fujita 1997; Solhaug et al. 2014; Kirilovsky 2015). This results in an excitation imbalance between the two photosystems in blue light (Luimstra et al. 2018). The PAL mutant lacks PBS. Therefore, if our hypothesis is correct, then the PAL mutant should have (i) a similar low biomass and O2 production rate and (ii) a similar low PSI:PSII ratio in both orange-red and blue light as the wildtype in blue light. This reasoning is confirmed by our experiments, in which the PAL mutant indeed displayed a similar low biomass (Fig. 1c), cell production (Fig. 1d), Chl a content (Fig. 1e), PSI:PSII ratio (Fig. 2c,d; Table 1), and O2 production rate per PSII (Fig. 3) in both orange-red and blue light as the wildtype in blue light. In other words, the poor photosynthetic performance of PBS-containing cyanobacteria in blue light was similar to the performance of a PBS-deficient mutant in both light colors.
We note that our results also show some differences between the wildtype in blue light and the PAL mutant. In particular, the photophysiology of the PAL mutant shows enhanced absorption in the 400–500 nm range (Fig. 2b), indicative of enhanced carotenoid production (see also Ajlani and Vernotte 1998; Kwon et al. 2013) and an even lower PSI:PSII ratio than the wildtype in blue light (Fig. 2d). Hence, in a sense, the PAL mutant lacking PBS might be interpreted as a more extreme phenotype in comparison to the wildtype that contains PBS but cannot use them in blue light.
The low growth rate and low PSI:PSII ratio of the PAL mutant have also been described by many other studies (Ajlani and Vernotte 1998; El Bissati and Kirilovsky 2001; Bernát et al. 2009; Stadnichuk et al. 2009; Collins et al. 2012; Kwon et al. 2013; Liberton et al. 2017). Comparison of different antenna mutants revealed that the PSI:PSII ratio decreases with a decrease in antenna size of the PBS, as a response to the diminished energy transfer from PBS to PSII when the antenna size is gradually reduced (Ajlani et al. 1995; Olive et al. 1997; Bernát et al. 2009; Stadnichuk et al. 2009; Collins et al. 2012; Kwon et al. 2013; Liberton et al. 2017). Likewise, Page et al. (2012) reported that growth rates were highest in the Synechocystis PCC 6803 wildtype, and decreased progressively with a decrease in antenna size of a series of antenna mutants. We also note the presence of the fluorescence peak at 685 nm in the 77 K fluorescence spectra of the PAL mutant (Fig. 2d), which has also been found in previously published 77 K spectra of the PAL mutant (Ajlani and Vernotte 1998; Bernát et al. 2009; Stadnichuk et al. 2009; Collins et al. 2012; Kwon et al. 2013). This peak, which is commonly attributed to the chlorophyll-binding protein CP43 in PSII (Andrizhiyevskaya et al. 2005; Wilson et al. 2007; Brecht et al. 2014), is also present in the wildtype in blue light (Fig. 2c). Hence, our findings with the PAL mutant are well in line with many previous studies. The novelty of our results is the recognition that, in many respects, the phenotype of the PAL mutant resembles the phenotype of the wildtype strain in blue light. This is further confirmed by our analysis of the oxygen production rates of the wildtype and PAL mutant, as measured by MIMS (Figs. 3, 4).
It has been shown that deletion of PBS in the PAL mutant is accompanied by profound changes in the proteome in comparison to the wildtype (Kwon et al. 2013; Liberton et al. 2017). Kwon and colleagues report a major increase of PSII proteins accompanied by a slight decrease of PSI proteins in the PAL mutant. Furthermore, they found that the PAL mutant has increased abundances of proteins involved in stress responses to high light and in carbohydrate metabolism (mostly gluconeogenesis). More recent proteome analyses by Liberton et al. (2017) support these findings, and in addition these authors found a decrease in bicarbonate transport proteins and an increase in transport proteins involved in nitrate uptake in the PAL mutant. Interestingly, the physiology of the wildtype is also completely changed when transferred from white to blue light (Singh et al. 2009). Transcriptome analysis by Singh et al. revealed that Synechocystis sp. PCC 6803 wildtype cells grown in blue light showed, among others, an increased expression of genes encoding PSII subunits and proteins involved in stress responses to high light, carbohydrate metabolism, and nitrate uptake, which resembles several of the proteome results in the PAL mutant (Kwon et al. 2013; Liberton et al. 2017). This indicates again that the PAL mutant and wildtype in blue light share many similarities, not only in their photophysiological traits but also in terms of their cellular metabolism.
Implications for biotechnology
Several studies have argued that truncation of light-harvesting antennae may be an advantage in crop and algal biomass production for, e.g., biotechnological applications (see, e.g., Ort and Melis 2011; Work et al. 2012; de Mooij et al. 2015; Kirst et al. 2017). In dense algal cultures or plant canopies, cells near the surface absorb excessive amounts of light energy that they largely dissipate as heat, while cells deeper down in the culture receive insufficient light for photosynthesis (Melis 2009; Blankenship and Chen 2013). As a consequence, much of the absorbed light energy is wasted rather than invested in biomass production. Truncation of the light-harvesting antennae reduces absorption per individual cell and thereby distributes light more evenly among the cells in dense algal cultures. It has indeed been demonstrated that antenna truncation can increase the biomass production of green algae (e.g., Polle et al. 2002; Perrine et al. 2012; de Mooij et al. 2015; Shin et al. 2016).
Our results indicate that truncation of the PBS of cyanobacteria has other effects than the truncation of the chlorophyll-based light-harvesting complexes of green algae. Green algae generally maintain a PSI:PSII ratio of around 1:1, in contrast to cyanobacteria which usually have a much higher PSI:PSII ratio (Shen et al. 1993; Singh et al. 2009; Kirilovsky 2015) and mainly use their PBS to direct sufficient light energy to PSII. Therefore, truncation of PBS in cyanobacteria will have a stronger effect on light redistribution between the two photosystems than truncation of the light-harvesting complex in green algae. In particular, our findings show that complete deletion of the PBS of cyanobacteria, in the PAL mutant, yields an excitation imbalance between the two photosystems, which results in a low PSII activity (Fig. 3) and low biomass production (Fig. 1) in both blue and orange-red light. This matches earlier results of, e.g., Ajlani and Vernotte (1998), Bernát et al. (2009), Page et al. (2012), and Kwon et al. (2013), who also found that the PAL mutant has a lower biomass production than the wildtype.
Possibly, partial truncation of the antennae by shortening or removing the rods of the PBS might improve the biomass production of cyanobacteria, as the PBS can then still distribute the absorbed light energy over the two photosystems while overall absorbance per cell is lower (Lea-Smith et al. 2014). Partial truncation of the PBS likely implies that less light will be transferred to PSII, which must be compensated by a decreased PSI:PSII ratio, to maintain the balance between the two photosystems. Several cyanobacterial studies show that partial truncation mutants indeed decreased their PSI:PSII ratio progressively and proportionally to a decrease in antenna size (Ajlani et al. 1995; Olive et al. 1997; Bernát et al. 2009; Stadnichuk et al. 2009; Collins et al. 2012; Kwon et al. 2013; Liberton et al. 2017). Although most of these mutants did not show a higher productivity than the wildtype, removal of the phycocyanin rods but not the allophycocyanin core in the Olive mutant (Rögner et al. 1990) resulted in an enhanced cyanobacterial productivity at high light intensities (Kwon et al. 2013) and low-carbon conditions (Lea-Smith et al. 2014).
Implications for cyanobacterial photosynthesis in the blue ocean
Marine cyanobacteria of the Synechococcus genus often use the phycobilin pigments phycoerythrobilin (PEB) and phycourobilin (PUB) to absorb wavelengths in the green part (525–575 nm) and blue-green part (475–515 nm) of the light spectrum (Six et al. 2007; Grébert et al. 2018). These phycobilin pigments do not absorb photons in the violet-blue part of the spectrum (≤ 450 nm), and hence, similar to Synechocystis PCC 6803, marine Synechococcus will be unable to use their PBS to harvest blue photons ≤ 450 nm for PSII (Luimstra et al. 2018). Consequently, Synechococcus usually thrives in the near-surface layers of the oceans and in coastal waters, where green and blue-green light is available (Partensky et al. 1999; Scanlan and West 2002; Stomp et al. 2007a).
Cyanobacteria of the genus Prochlorococcus abound in the subtropical ocean gyres and usually their populations extend deeper down in the water column than Synechococcus (Partensky et al. 1999; Scanlan and West 2002). They use a different light-harvesting strategy. Prochlorococcus does not deploy PBS, but has evolved light-harvesting antennae that effectively absorb blue light using divinyl-Chl a and b (Chisholm et al. 1992; Ting et al. 2002; Stomp et al. 2007b). Prochlorococcus likely evolved from a PBS-containing cyanobacterium (Kettler et al. 2007; Scanlan et al. 2009), and some Prochlorococcus strains still have genes for the synthesis of phycoerythrin (Hess et al. 1996). Interestingly, our results show that the loss of PBS does not have major negative fitness consequences in habitats dominated by blue light, as the PAL mutant had a similarly low biomass and cell production in blue light as the wildtype. Furthermore, we note that the 685-nm peak in our 77 K fluorescence spectra, indicative of the chlorophyll-binding protein CP43, was strongly induced by blue light (Fig. 2c) and in the PBS-deficient mutant (Fig. 2d; see also Ajlani and Vernotte 1998; Kwon et al. 2013). The chlorophyll-binding proteins (Pcb) in the light-harvesting antennae of Prochlorococcus are closely related to CP43 (Chen and Bibby 2005). Our results therefore suggest that the loss of PBS and transformation from an ancestral chlorophyll-binding protein of the CP43 family to Pcb caused a major evolutionary transition, from the poor photosynthetic performance in blue light by PBS-based cyanobacteria to efficient utilization of blue light by the chlorophyll-based light-harvesting antennae of Prochlorococcus. Harnessing of blue light will have provided a major selective advantage for Prochlorococcus over PBS-containing cyanobacteria in the deep blue waters of the open ocean.
Acknowledgements
We are most grateful to our late colleague Dr. Hans Matthijs for initiating this research. Synechocystis sp. PCC 6803 strains were kindly provided by Devaki Bhaya (wildtype) and Ghada Ajlani (PAL mutant). We thank Hugo Pineda Hernández for help with R, participants of the Wetsus research theme Algae and the FAME department of the University of Amsterdam for fruitful discussions, and two anonymous reviewers for useful comments on the manuscript. This work has been performed in the cooperation framework of Wetsus, European Centre of Excellence for Sustainable Water Technology (http://www.wetsus.eu). Wetsus is co-funded by the Dutch Ministry of Economic Affairs and Ministry of Infrastructure and Environment, the Province of Fryslân and the Northern Netherlands Provinces.
Author Contributions
VML, JMS, and HCPM conceived the original research plan and designed the experiments; VML and CFMC performed the experiments and analyzed the data, with technical assistance from JMS and scientific supervision by KJH and JH; all authors except HCPM contributed to the writing of the manuscript, and VML and JH wrote the final version.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Hans C.P. Matthijs—Deceased, 17 April 2016.
References
- Ajlani G, Vernotte C. Construction and characterization of a phycobiliprotein-less mutant of Synechocystis sp. PCC 6803. Plant Mol Biol. 1998;37:577–580. doi: 10.1023/A:1005924730298. [DOI] [PubMed] [Google Scholar]
- Ajlani G, Vernotte C, DiMagno L, Haselkorn R. Phycobilisome core mutants of Synechocystis PCC 6803. Biochim Biophys Acta. 1995;1231:189–196. doi: 10.1016/0005-2728(95)00086-X. [DOI] [Google Scholar]
- Allahverdiyeva Y, Suorsa M, Tikkanen M, Aro EM. Photoprotection of photosystems in fluctuating light intensities. J Exp Bot. 2014;66:2427–2436. doi: 10.1093/jxb/eru463. [DOI] [PubMed] [Google Scholar]
- Ananyev G, Dismukes GC. How fast can photosystem II split water? Kinetic performance at high and low frequencies. Photosynth Res. 2005;84:355–365. doi: 10.1007/s11120-004-7081-1. [DOI] [PubMed] [Google Scholar]
- Andrizhiyevskaya EG, Chojnicka A, Bautista JA, Diner BA, van Grondelle R, Dekker JP. Origin of the F685 and F695 fluorescence in photosystem II. Photosynth Res. 2005;84:173–180. doi: 10.1007/s11120-005-0478-7. [DOI] [PubMed] [Google Scholar]
- Bañares-España E, Kromkamp JC, López-Rodas V, Costas E, Flores-Moya A. Photoacclimation of cultured strains of the cyanobacterium Microcystis aeruginosa to high-light and low-light conditions. FEMS Microbiol Ecol. 2013;83:700–710. doi: 10.1111/1574-6941.12025. [DOI] [PubMed] [Google Scholar]
- Bernát G, Waschewski N, Rögner M. Towards efficient hydrogen production: the impact of antenna size and external factors on electron transport dynamics in Synechocystis PCC 6803. Photosynth Res. 2009;99:205–216. doi: 10.1007/s11120-008-9398-7. [DOI] [PubMed] [Google Scholar]
- Bland E, Angenent LT. Pigment-targeted light wavelength and intensity promotes efficient photoautotrophic growth of Cyanobacteria. Biores Technol. 2016;216:579–586. doi: 10.1016/j.biortech.2016.05.116. [DOI] [PubMed] [Google Scholar]
- Blankenship RE, Chen M. Spectral expansion and antenna reduction can enhance photosynthesis for energy production. Curr Opin Chem Biol. 2013;17:457–461. doi: 10.1016/j.cbpa.2013.03.031. [DOI] [PubMed] [Google Scholar]
- Brecht M, Skandary S, Hellmich J, Glöckner C, Konrad A, Hussels M, Meixner AJ, Zouni A, Schlodder E. Spectroscopic properties of photosystem II core complexes from Thermosynechococcus elongatus revealed by single-molecule experiments. Biochim Biophys Acta. 2014;1837:773–781. doi: 10.1016/j.bbabio.2014.01.025. [DOI] [PubMed] [Google Scholar]
- Chen M, Bibby TS. Photosynthetic apparatus of antenna-reaction centres supercomplexes in oxyphotobacteria: insight through significance of Pcb/IsiA proteins. Photosynth Res. 2005;86:165–173. doi: 10.1007/s11120-005-1330-9. [DOI] [PubMed] [Google Scholar]
- Chisholm SW, Frankel SL, Goericke R, Olson RJ, Palenik B, Waterbury JB, West-Johnsrud L, Zettler ER. Prochlorococcus marinus nov. gen. nov. sp.: an oxyphototrophic marine prokaryote containing divinyl chlorophyll a and b. Arch Microbiol. 1992;157:297–300. doi: 10.1007/BF00245165. [DOI] [Google Scholar]
- Collins AM, Liberton M, Jones HD, Garcia OF, Pakrasi HB, Timlin JA. Photosynthetic pigment localization and thylakoid membrane morphology are altered in Synechocystis 6803 phycobilisome mutants. Plant Physiol. 2012;158:1600–1609. doi: 10.1104/pp.111.192849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Mooij T, Janssen M, Cerezo-Chinarro O, Mussgnug JH, Kruse O, Ballottari M, Bassi R, Bujaldon S, Wollman F, Wijffels RH. Antenna size reduction as a strategy to increase biomass productivity: a great potential not yet realized. J Appl Phycol. 2015;27:1063–1077. doi: 10.1007/s10811-014-0427-y. [DOI] [Google Scholar]
- Du W, Jongbloets JA, Hernández HP, Bruggeman FJ, Hellingwerf KJ, Branco dos Santos F. Photonfluxostat: a method for light-limited batch cultivation of cyanobacteria at different, yet constant, growth rates. Algal Res. 2016;20:118–125. doi: 10.1016/j.algal.2016.10.004. [DOI] [Google Scholar]
- El Bissati K, Kirilovsky D. Regulation of psbA and psaE expression by light quality in Synechocystis species PCC 6803: a redox control mechanism. Plant Physiol. 2001;125:1988–2000. doi: 10.1104/pp.125.4.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita Y. A study on the dynamic features of photosystem stoichiometry: accomplishments and problems for future studies. Photosynth Res. 1997;53:83–93. doi: 10.1023/A:1005870301868. [DOI] [Google Scholar]
- Grébert T, Doré H, Partensky F, Farrant GK, Boss ES, Picheral M, Guidi L, Pesant S, Scanlan DJ, Wincker P, Acinas SG, Kehoe DM, Garczarek L. Light color acclimation is a key process in the global ocean distribution of Synechococcus cyanobacteria. Proc Natl Acad Sci USA. 2018;115:E2010–E2019. doi: 10.1073/pnas.1717069115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossman AR, Schaefer MR, Chiang GG, Collier JL. The phycobilisome, a light-harvesting complex responsive to environmental conditions. Microbiol Rev. 1993;57:725–749. doi: 10.1128/mr.57.3.725-749.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hess WR, Partensky F, Van der Staay GWM, Garcia-Fernandez JM, Börner T, Vaulot D. Coexistence of phycoerythrin and a chlorophyll a/b antenna in a marine prokaryote. Proc Natl Acad Sci USA. 1996;93:11126–11130. doi: 10.1073/pnas.93.20.11126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland HD. Volcanic gases, black smokers, and the Great Oxidation Event. Geochim Cosmochim Acta. 2002;66:3811–3826. doi: 10.1016/S0016-7037(02)00950-X. [DOI] [Google Scholar]
- Huisman J, Matthijs HCP, Visser PM, Balke H, Sigon CAM, Passarge J, Weissing FJ, Mur LR. Principles of the light-limited chemostat: theory and ecological applications. Antonie Leeuwenhoek. 2002;81:117–133. doi: 10.1023/A:1020537928216. [DOI] [PubMed] [Google Scholar]
- Jordan P, Fromme P, Witt HT, Klukas O, Saenger W, Krauß N. Three-dimensional structure of cyanobacterial photosystem I at 2.5 Å resolution. Nature. 2001;411:909–917. doi: 10.1038/35082000. [DOI] [PubMed] [Google Scholar]
- Joshua S, Bailey S, Mann NH, Mullineaux CW. Involvement of phycobilisome diffusion in energy quenching in cyanobacteria. Plant Physiol. 2005;138:1577–1585. doi: 10.1104/pp.105.061168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kana TM, Darkangelo C, Hunt MD, Oldham JB, Bennett GE, Cornwell JC. Membrane inlet mass spectrometer for rapid high-precision determination of N2, O2, and Ar in environmental water samples. Anal Chem. 1994;66:4166–4170. doi: 10.1021/ac00095a009. [DOI] [Google Scholar]
- Kettler GC, Martiny AC, Huang K, Zucker J, Coleman ML, Rodrigue S, Chen F, Lapidus A, Ferriera S, Johnson J, Steglich C, Church GM, Richardson P, Chisholm SW. Patterns and implications of gene gain and loss in the evolution of Prochlorococcus. PLoS Genet. 2007;3:e231. doi: 10.1371/journal.pgen.0030231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirilovsky D. Modulating energy arriving at photochemical reaction centers: orange carotenoid protein-related photoprotection and state transitions. Photosynth Res. 2015;126:3–17. doi: 10.1007/s11120-014-0031-7. [DOI] [PubMed] [Google Scholar]
- Kirst H, Gabilly ST, Niyogi KK, Lemaux PG, Melis A. Photosynthetic antenna engineering to improve crop yields. Planta. 2017;245:1009–1020. doi: 10.1007/s00425-017-2659-y. [DOI] [PubMed] [Google Scholar]
- Kwon J, Bernát G, Wagner H, Rögner M, Rexroth S. Reduced light-harvesting antenna: consequences on cyanobacterial metabolism and photosynthetic productivity. Algal Res. 2013;2:188–195. doi: 10.1016/j.algal.2013.04.008. [DOI] [Google Scholar]
- Lea-Smith DJ, Bombelli P, Dennis JS, Scott SA, Smith AG, Howe CJ. Phycobilisome-deficient strains of Synechocystis sp. PCC 6803 have reduced size and require carbon-limiting conditions to exhibit enhanced productivity. Plant Physiol. 2014;165:705–714. doi: 10.1104/pp.114.237206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemasson C, Tandeau de Marsac N, Cohen-Bazire G. Role of allophycocyanin as light-harvesting pigment in cyanobacteria. Proc Natl Acad Sci USA. 1973;70:3130–3133. doi: 10.1073/pnas.70.11.3130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liberton M, Chrisler WB, Nicora CD, Moore RJ, Smith RD, Koppenaal DW, Pakrasi HB, Jacobs JM. Phycobilisome truncation causes widespread proteome changes in Synechocystis sp. PCC 6803. PLoS One. 2017;12:e0173251. doi: 10.1371/journal.pone.0173251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luimstra VM, Schuurmans JM, Verschoor AM, Hellingwerf KJ, Huisman J, Matthijs HCP. Blue light reduces photosynthetic efficiency of cyanobacteria through an imbalance between photosystems I and II. Photosynth Res. 2018;138:177–189. doi: 10.1007/s11120-018-0561-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao HB, Li GF, Li DH, Wu QY, Gong YD, Zhang XF, Zhao NM. Effects of glycerol and high temperatures on structure and function of phycobilisomes in Synechocystis sp. PCC 6803. FEBS Lett. 2003;553:68–72. doi: 10.1016/S0014-5793(03)00973-6. [DOI] [PubMed] [Google Scholar]
- Melis A. Solar energy conversion efficiencies in photosynthesis: minimizing the chlorophyll antennae to maximize efficiency. Plant Sci. 2009;177:272–280. doi: 10.1016/j.plantsci.2009.06.005. [DOI] [Google Scholar]
- Mullineaux CW. Electron transport and light-harvesting switches in cyanobacteria. Front Plant Sci. 2014;5:7. doi: 10.3389/fpls.2014.00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murakami A. Quantitative analysis of 77K fluorescence emission spectra in Synechocystis sp. PCC 6714 and Chlamydomonas reinhardtii with variable PSI/PSII stoichiometries. Photosynth Res. 1997;53:141–148. doi: 10.1023/A:1005818317797. [DOI] [Google Scholar]
- Myers J, Graham JR, Wang RT. Light harvesting in Anacystis nidulans studied in pigment mutants. Plant Physiol. 1980;66:1144–1149. doi: 10.1104/pp.66.6.1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olive J, Ajlani G, Astier C, Recouvreur M, Vernotte C. Ultrastructure and light adaptation of phycobilisome mutants of Synechocystis PCC 6803. Biochim Biophys Acta. 1997;1319:275–282. doi: 10.1016/S0005-2728(96)00168-5. [DOI] [Google Scholar]
- Ort DR, Melis A. Optimizing antenna size to maximize photosynthetic efficiency. Plant Physiol. 2011;155:79–85. doi: 10.1104/pp.110.165886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page LE, Liberton M, Pakrasi HB. Reduction of photoautotrophic productivity in the cyanobacterium Synechocystis sp. strain PCC 6803 by phycobilisome antenna truncation. Appl Environ Microbiol. 2012;78:6349–6351. doi: 10.1128/AEM.00499-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Partensky F, Hess WR, Vaulot D. Prochlorococcus, a marine photosynthetic prokaryote of global significance. Microbiol Mol Biol Rev. 1999;63:106–127. doi: 10.1128/mmbr.63.1.106-127.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrine Z, Negi S, Sayre RT. Optimization of photosynthetic light energy utilization by microalgae. Algal Res. 2012;1:134–142. doi: 10.1016/j.algal.2012.07.002. [DOI] [Google Scholar]
- Platt T, Jassby AD. The relationship between photosynthesis and light for natural assemblages of coastal marine phytoplankton. J Phycol. 1976;12:421–430. [Google Scholar]
- Polle JEW, Kanakagiri S, Jin ES, Masuda T, Melis A. Truncated chlorophyll antenna size of the photosystems: a practical method to improve microalgal productivity and hydrogen production in mass culture. Int J Hydrogen Energy. 2002;27:1257–1264. doi: 10.1016/S0360-3199(02)00116-7. [DOI] [Google Scholar]
- Porra RJ, Thompson WA, Kriedemann PE. Determination of accurate extinction coefficients and simultaneous equations for assaying chlorophylls a and b extracted with four different solvents: verification of the concentration of chlorophyll standards by atomic absorption spectroscopy. Biochim Biophys Acta Bioeng. 1989;975:384–394. doi: 10.1016/S0005-2728(89)80347-0. [DOI] [Google Scholar]
- Pulich WM, van Baalen C. Growth requirements of blue-green algae under blue light conditions. Arch Microbiol. 1974;97:303–312. doi: 10.1007/BF00403069. [DOI] [Google Scholar]
- R Development Core Team . R: a language and environment for statistical computing. Vienna: R Foundation for Statistical Computing; 2017. [Google Scholar]
- Rakhimberdieva MG, Boichenko VA, Karapetyan NV, Stadnichuk IN. Interaction of phycobilisomes with photosystem II dimers and photosystem I monomers and trimers in the cyanobacterium Spirulina platensis. Biochem. 2001;40:15780–15788. doi: 10.1021/bi010009t. [DOI] [PubMed] [Google Scholar]
- Rögner M, Nixon PJ, Diner BA. Purification and characterization of photosystem I and photosystem II core complexes from wild-type and phycocyanin-deficient strains of the cyanobacterium Synechocystis PCC 6803. J Biol Chem. 1990;265:6189–6196. [PubMed] [Google Scholar]
- Scanlan DJ, West NJ. Molecular ecology of the marine cyanobacterial genera Prochlorococcus and Synechococcus. FEMS Microbiol Ecol. 2002;40:1–12. doi: 10.1111/j.1574-6941.2002.tb00930.x. [DOI] [PubMed] [Google Scholar]
- Scanlan DJ, Ostrowski M, Mazard S, Dufresne A, Garczarek L, Hess WR, Post AF, Hagemann M, Paulsen I, Partensky F. Ecological genomics of marine picocyanobacteria. Microbiol Mol Biol Rev. 2009;73:249–299. doi: 10.1128/MMBR.00035-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schirrmeister BE, Gugger M, Donoghue PC. Cyanobacteria and the Great Oxidation Event: evidence from genes and fossils. Palaeontology. 2015;58:769–785. doi: 10.1111/pala.12178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuurmans RM, Matthijs HCP, Hellingwerf KJ. Transition from exponential to linear photoautotrophic growth changes the physiology of Synechocystis sp. PCC 6803. Photosynth Res. 2017;132:69–82. doi: 10.1007/s11120-016-0329-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen G, Boussiba S, Vermaas WF. Synechocystis sp PCC 6803 strains lacking photosystem I and phycobilisome function. Plant Cell. 1993;5:1853–1863. doi: 10.1105/tpc.5.12.1853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin W, Lee B, Jeong B, Chang YK, Kwon J. Truncated light-harvesting chlorophyll antenna size in Chlorella vulgaris improves biomass productivity. J Appl Phycol. 2016;28:3193–3202. doi: 10.1007/s10811-016-0874-8. [DOI] [Google Scholar]
- Singh AK, Bhattacharyya-Pakrasi M, Elvitigala T, Ghosh B, Aurora R, Pakrasi HB. A systems-level analysis of the effects of light quality on the metabolism of a cyanobacterium. Plant Physiol. 2009;151:1596–1608. doi: 10.1104/pp.109.144824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Six C, Thomas J, Garczarek L, Ostrowski M, Dufresne A, Blot N, Scanlan DJ, Partensky F. Diversity and evolution of phycobilisomes in marine Synechococcus spp.: a comparative genomics study. Genome Biol. 2007;8:R259. doi: 10.1186/gb-2007-8-12-r259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solhaug KA, Xie L, Gauslaa Y. Unequal allocation of excitation energy between photosystem II and I reduces cyanolichen photosynthesis in blue light. Plant Cell Physiol. 2014;55:1404–1414. doi: 10.1093/pcp/pcu065. [DOI] [PubMed] [Google Scholar]
- Stadnichuk IN, Lukashev EP, Elanskaya IV. Fluorescence changes accompanying short-term light adaptations in photosystem I and photosystem II of the cyanobacterium Synechocystis sp. PCC 6803 and phycobiliprotein-impaired mutants: State 1/State 2 transitions and carotenoid-induced quenching of phycobilisomes. Photosynth Res. 2009;99:227–241. doi: 10.1007/s11120-009-9402-x. [DOI] [PubMed] [Google Scholar]
- Stamatakis K, Tsimilli-Michael M, Papageorgiou GC. On the question of the light-harvesting role of β-carotene in photosystem II and photosystem I core complexes. Plant Physiol Biochem. 2014;81:121–127. doi: 10.1016/j.plaphy.2014.01.014. [DOI] [PubMed] [Google Scholar]
- Stomp M, Huisman J, Vörös L, Pick FR, Laamanen M, Haverkamp T, Stal LJ. Colourful coexistence of red and green picocyanobacteria in lakes and seas. Ecol Lett. 2007;10:290–298. doi: 10.1111/j.1461-0248.2007.01026.x. [DOI] [PubMed] [Google Scholar]
- Stomp M, Huisman J, Stal LJ, Matthijs HCP. Colorful niches of phototrophic microorganisms shaped by vibrations of the water molecule. ISME J. 2007;1:271–282. doi: 10.1038/ismej.2007.59. [DOI] [PubMed] [Google Scholar]
- Tandeau de Marsac N. Phycobiliproteins and phycobilisomes: the early observations. Photosynth Res. 2003;76:193–205. doi: 10.1023/A:1024954911473. [DOI] [PubMed] [Google Scholar]
- Ting CS, Rocap G, King J, Chisholm SW. Cyanobacterial photosynthesis in the oceans: the origins and significance of divergent light-harvesting strategies. Trends Microbiol. 2002;10:134–142. doi: 10.1016/S0966-842X(02)02319-3. [DOI] [PubMed] [Google Scholar]
- Tyystjärvi T, Tuominen I, Herranen M, Aro E, Tyystjärvi E. Action spectrum of psbA gene transcription is similar to that of photoinhibition in Synechocystis sp. PCC 6803. FEBS Lett. 2002;516:167–171. doi: 10.1016/S0014-5793(02)02537-1. [DOI] [PubMed] [Google Scholar]
- Umena Y, Kawakami K, Shen J, Kamiya N. Crystal structure of oxygen-evolving photosystem II at a resolution of 1.9 Å. Nature. 2011;473:55. doi: 10.1038/nature09913. [DOI] [PubMed] [Google Scholar]
- Wang CY, Fu CC, Liu YC. Effects of using light-emitting diodes on the cultivation of Spirulina platensis. Biochem Eng J. 2007;37:21–25. doi: 10.1016/j.bej.2007.03.004. [DOI] [Google Scholar]
- Wilde A, Churin Y, Schubert H, Börner T. Disruption of a Synechocystis sp. PCC 6803 gene with partial similarity to phytochrome genes alters growth under changing light qualities. FEBS Lett. 1997;406:89–92. doi: 10.1016/S0014-5793(97)00248-2. [DOI] [PubMed] [Google Scholar]
- Wilson A, Boulay C, Wilde A, Kerfeld CA, Kirilovsky D. Light-induced energy dissipation in iron-starved cyanobacteria: roles of OCP and IsiA proteins. Plant Cell. 2007;19:656–672. doi: 10.1105/tpc.106.045351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Work VH, D’Adamo S, Radakovits R, Jinkerson RE, Posewitz MC. Improving photosynthesis and metabolic networks for the competitive production of phototroph-derived biofuels. Curr Opin Biotechnol. 2012;23:290–297. doi: 10.1016/j.copbio.2011.11.022. [DOI] [PubMed] [Google Scholar]