Figure 5.
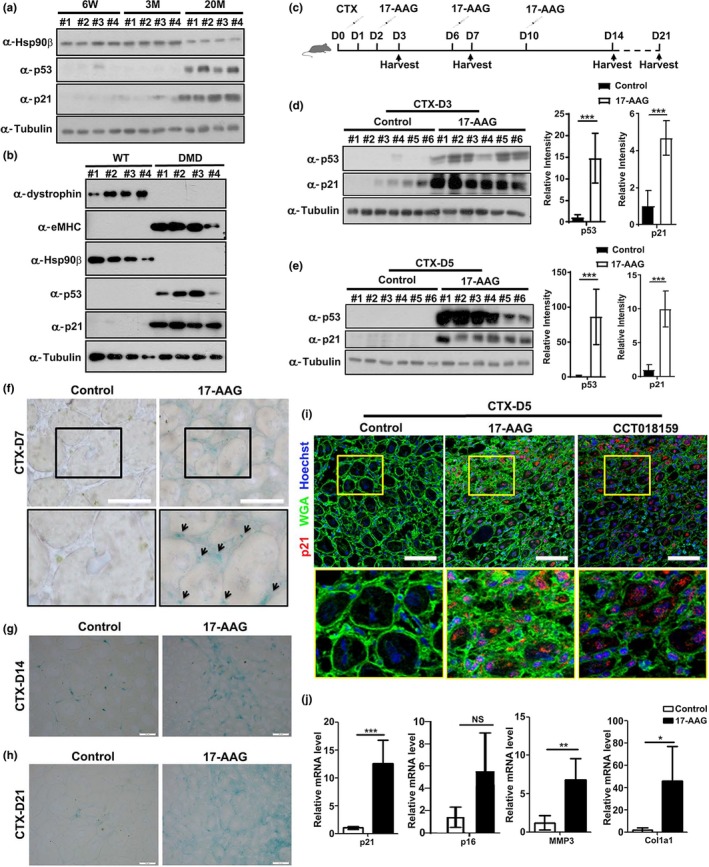
Hsp90 inhibitor 17‐AAG induced p53‐dependent senescence in vivo. (a) TA muscles from four (#1‐#4) 6‐week‐old (6W), 3‐month‐old (3M), or 20‐month‐old (20M) male mice were subjected to Western blot analysis to determine the expression levels of Hsp90β, p53, and p21. (b) TA muscles from four (#1‐#4) control or DMD male mice were subjected to Western blot analysis to determine the expression levels of Hsp90β, p53, p21, and eMHC. (c) Injection scheme for control DMSO or 17‐AAG injection into CTX‐injured mouse TA muscles. (d) TA muscles with DMSO or 17‐AAG injection from six male mice (#1‐#6) for each group were harvested on day 3 post‐CTX injury for Western blot analysis. The expression levels of p53 and p21 were quantified. (e) TA muscles with DMSO or 17‐AAG injection from six male mice (#1‐#6) for each group were harvested on day 5 post‐CTX injury for Western blot analysis. The expression levels of p53 and p21 were quantified. (f) Cryosections of regenerating TA muscles on day 7 post‐CTX injury from control or 17‐AAG‐injected male mice were subjected to SA‐βGal staining. Bar: 50μm. (g) Cryosections of regenerating TA muscles on day 14 post‐CTX injury from control or 17‐AAG‐injected male mice were subjected to SA‐βGal staining. Bar: 50μm. (h) Cryosections of regenerating TA muscles on day 21 post‐CTX injury from control or 17‐AAG‐injected male mice were subjected to SA‐βGal staining. Bar: 50μm. (i) Cryosections of regenerating TA muscles on day 5 post‐CTX injury from control, 17‐AAG‐, or CTT018159‐injected male mice were subjected to p21 staining. Bar: 50μm. (j) Cryosections of regenerating TA muscles on day 14 post‐CTX injury from control or 17‐AAG‐injected mice were subjected to RT–qPCR analysis for expression of p21, p16, MMP3, and Collagen I. Data shown are representative of three biological replicates. Statistical analysis was performed with Student's t test (*p < 0.05, **p < 0.01, and ***p < 0.001)
