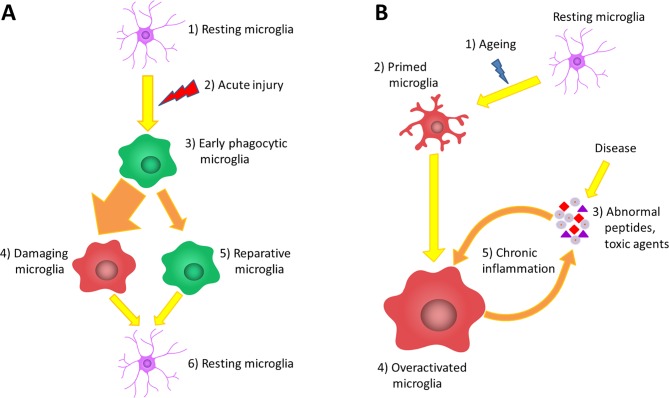
Figure 1.
The role of microglia in acute and chronic inflammations. (A) Under normal circumstances, the brain tissue is constantly monitored by resting microglia cells (1). Acute brain damage triggers dynamic microglia activation. This is a linear process that will decline over time (2). First, an early phagocytic phenotype accumulates in the injured area and begins to remove the debris (3). However, the majority of microglia rapidly converts to a pro-inflammatory phenotype via classical activation, which is neurotoxic, induces apoptosis and exacerbates inflammatory processes (4). At the same time, some microglia get alternatively activated and exert a neuroprotective effect to counteract the pro-inflammatory processes (5). After the resolution of the inflammatory cascades, microglia return to resting state and continue the tissue surveillance (6). (B) Due to physiological aging, the basic activation state of microglia is increased (2), which is associated with elevated production of cytokines and inflammatory mediators. These so-called primed microglia cells show an elevated responsiveness (3) and potentially can be more stimulated by binding disease-associated proteins and other toxic agents (4). The over-activation results in the impairment of certain microglial functions lowering their ability to eliminate the toxic protein aggregates; however, in line with their phagocytic dysfunction, their productivity of inflammatory cytokines further increases (5). This negative correlation between the expression of pro-inflammatory cytokines and the clearance of abnormal proteins generates the vicious cycle of chronic inflammation, which is a characteristic of neurodegenerative diseases (6).