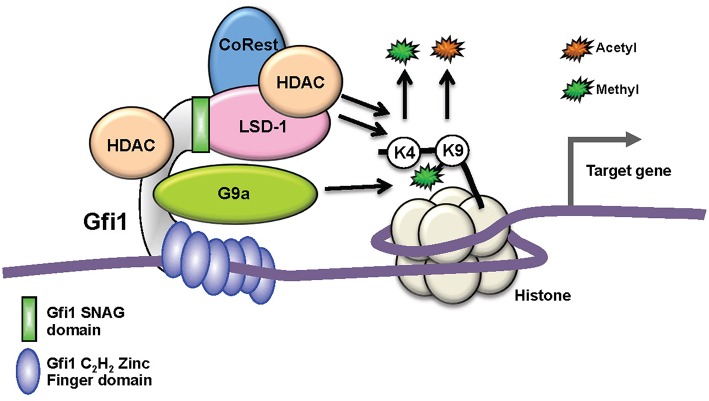
Figure 1.
Biochemical function of GFI1 as a transcription factors. Schematic depiction of GFI1 in a complex with its interacting proteins occupying a genomic site 5′ of a target gene. Whereas, the recruitment of the LSD1/CoRest complex occurs through the N-terminal SNAG domain (green), the middle domain (gray) serves mainly as an interaction platform for other proteins and the C2H2 zinc finger domains (blue) mediate DNA binding. G9A and HDACs can bind directly to GFI1, but HDACs can also be part of the LSD1/CoRest complex. HDACs and LSD recruitment leads to the removal of acetyl- or methyl groups from Histone H3 lysine 9 or 4, respectively. G9A enables the methylation of H3 lysine 9.