Abstract
The global burden of hepatitis B virus (HBV) infection is profound, and represents a public health threat as chronic infection can lead to liver cirrhosis, hepatocellular carcinoma, and death. The risk factors for chronic hepatitis B-related liver disease differ according to HBV endemicity, hepatitis B e-antigen seropositivity, and viral load. It is important to identify these risk factors and start antiviral treatment at an appropriate time according to current guidelines. The most crucial step in reducing HBV infection is prevention in infancy or early childhood, as infection at an early stage may lead to chronicity. South Korea was formerly an HBV-endemic area, but the epidemiology of HBV infection was changed by the introduction of vaccination in 1983 and nationwide immunization in 1995. The government and the private sector made efforts to reduce the prevalence of HBV infection, and Korea is on target to meet the World Health Organization goal of eliminating viral hepatitis by 2030. To eliminate hepatitis worldwide, the costs of antiviral treatment to prevent perinatal HBV transmission in pregnant women with high viremia should be covered by a national program, and strategies to reduce the prevalence of HBV infection in immigrant populations are needed.
Keywords: Hepatitis B, chronic; Vaccination; Epidemiology
INTRODUCTION
The World Health Organization (WHO) Global Strategy for Viral Hepatitis was approved during the World Health Assembly in May 2016. Hepatitis was elevated to a higher priority with a goal of eliminating viral hepatitis as a public health threat by 2030. The goal of the WHO is to achieve a 90% reduction in the incidence of chronic hepatitis B (CHB) and C infection and a 65% reduction in the mortality rate of viral hepatitis by 2030. Therefore, it is necessary to understand the current status of, and establish reliable strategies to eliminate, hepatitis B virus (HBV) infection in Korea.
South Korea is an intermediate endemic area for HBV infection; the estimated prevalence was approximately 3% in the 2016 Korea National Health and Nutrition Examination Survey (KNHANES) [1]. Chronic HBV infection is a major cause of liver cirrhosis and hepatocellular carcinoma (HCC) [2]. However, dramatic progress has been made in the management of chronic HBV infection after the introduction of HBV vaccination, nationwide screening and advances in antiviral treatment [3]. Here we review the changing epidemiology and burden of HBV infection and strategies to control HBV infection in Korea.
EPIDEMIOLOGY
Chronic hepatitis B prevalence
The WHO estimated that in 2015, 257 million persons or 3.5% of the population were living with chronic HBV infection. Among HBV-infected patients, approximately 27 million (10.5%) were aware of their infection and an estimated 4.5 million (16.7%) of them were on treatment. The prevalence of HBV infection differs geographically: 6% in Africa, 2% in Southeast Asia, and 1% in the Americas [4].
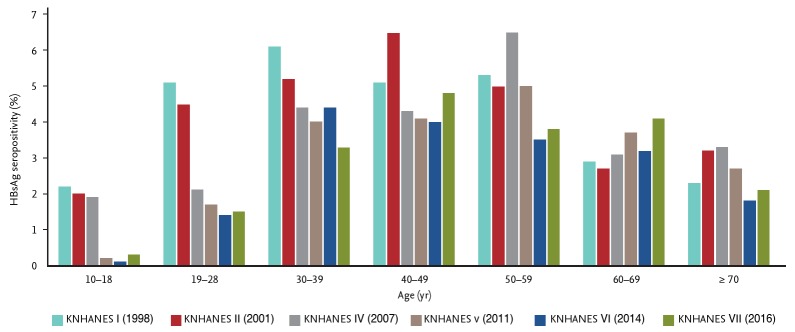
HBV endemicity differs according to the mode of infection (Table 1) [5]. Countries with a high prevalence have perinatal transmission as the major mode of infection; while low prevalence was due to horizontal transmission. Transmission by both modes is important in countries with an intermediate prevalence, such as South Korea, due to vaccination, perinatal care, and public health. The HBV disease burden in Korea results mainly from infections acquired through perinatal transmission. However, after implementation of a vaccination program in 1983, Korea was classified as an area of intermediate endemicity. The prevalence of hepatitis B surface antigen (HBsAg) carriage was 8% to 10% in the 1980s, 4.6% in the 1990s, and has remained at 2.9% since 2010. HBV vaccination for all newborns was introduced in 1983 (coverage rate, 79.7%), and the Expanded Program on Immunization (EPI) was introduced in 1995 (coverage rate, 98.9%) [1,6]. The HBsAg-positivity rates of younger age groups markedly declined to 2.2% in 1998, 1.9% in 2001, 1.9% in 2007, and 0.3% in 2016. The prevalence of HBsAg carriage also decreased significantly among those in their 20s and 30s (Fig. 1). The prevalence of CHB in Korean children has met the WHO interim 2020 target, 1% in children aged 5 years, and is projected to decline to 0.1% by 2030.
Table 1.
Levels of hepatitis B virus endemicity
| Types of endemicity | HBsAg prevalence, % | Mode of infection | Geographic distribution |
|---|---|---|---|
| High | ≥ 8 | Perinatal transmission | Parts of sub-Saharan Africa |
| Intermediate | 2–7 | Perinatal and horizontal transmission | Eastern Europe; Central Asia; Southeast Asia; China; Japan |
| Low | < 2 | Horizontal transmission | United States; Western Europe |
Adapted from World Health Organization [5].
HBsAg, hepatitis B surface antigen.
Figure 1.
Changes in the prevalence of hepatitis B virus carriage according to age group. Adapted from Korea Centers for Disease Control & Prevention [1]. HBsAg, hepatitis B surface antigen; KNHANES, Korea National Health and Nutrition Examination Survey.
The overall HBsAg positivity rate was slightly higher in men than in women, but the difference gradually decreased from 1.1% in 1998 to 0.3% in 2016. In 2016, the rate was highest in men and women in their 40s and 50s (5.7% and 4.7%, respectively) (Fig. 2) [1]. HBsAg seropositivity did not differ between low (2.6%) and high income (2.4%) groups or between urban (2.9%) and rural (2.5%) residents [1].
Figure 2.

Prevalence of hepatitis B virus carriage according to age and gender. Adapted from Korea Centers for Disease Control & Prevention [1].
Incidence of acute hepatitis B
The trends of acute hepatitis B changed in response to implementation of the HBV vaccination program. The incidence of acute viral hepatitis A and B among overall acute hepatitis in the early 1990s was 3.4% and 60.3%, respectively, with HBV infection as the most common cause of acute hepatitis [7]. In 2008, the incidence of acute HBV infection decreased to 5%, while that of acute hepatitis A virus infection increased to 70% [8]. The most frequently infected age group was 10 to 29 years of age in the 1980s, and 30 to 39 years of age in the early 2000s (Fig. 3) [9]. Approximately 5% of all acute HBV infections progress to chronic infection [10].
Figure 3.
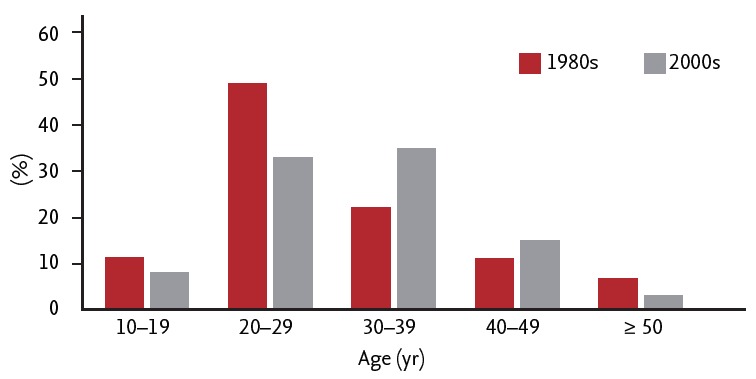
Age distribution of acute hepatitis B patients in the 1980s and 2000s. Adapted from Yim et al. [8] and Choi et al. [9].
In patients who recover from acute hepatitis B, HBV replication may persist in some organs, most likely the liver or peripheral blood cells, for a long period despite the presence of serum antibodies to HBsAg (anti-HBs) and HBV-specific cytotoxic T-cells [11,12]. HBV DNA was detected in the liver tissues of 13 of 14 healthy liver transplant donors who were positive for hepatitis B core antibody (anti-HBc) and anti-HBs [13]. Persistent histological abnormalities, including fibrosis and mild inflammation, were present for 10 years in another study, despite complete serological recovery after acute infection [14].
HBsAg-negative but anti-HBc-positive patients are at risk of HBsAg reversion, particularly during rituximab-based chemotherapy. Therefore, HBsAg and anti-HBc should be monitored in patients who are candidates for rituximab-based chemotherapy or bone marrow transplantation [15-17]. Because HBV transmission may still occur in unvaccinated and uninfected adults and can lead to chronicity, “catch-up” HBV vaccination should be given to prevent acute HBV infection in individuals who were not vaccinated in early childhood.
BURDEN OF CHRONIC HEPATITIS B VIRUS INFECTION
Disease course
The frequency of complications varies geographically. In low-endemic areas, the incidence of liver-related complications in untreated, asymptomatic patients can be as low as 1.2% after 16 years [18]. By contrast, the 5-year cumulative incidence of cirrhosis in East Asian (Taiwan and Korea) patients with hepatitis B e-antigen (HBeAg)-positive and -negative hepatitis was 8% to 17% and 13% to 38%, respectively. The 5-year cumulative incidence of liver decompensation among patients with cirrhosis was 15%. The incidence of HCC differs according to liver disease status; the 5-year cumulative incidence was 1% in inactive carriage, 3% in CHB patients without cirrhosis, and 17% in those with cirrhosis [19]. The 5-year cumulative incidence of survival in patients with compensated and decompensated cirrhosis was 85% and 14% to 35%, respectively [19-21]. The prevalence was similar in Korea before the era of antiviral agents, and the predominant cause of HCC was HBV infection (62% to 75% of HCC) [22].
After the introduction of oral antiviral therapy in 1999, the epidemiology of liver disease and liver cancer in Korea changed [23]. Real-world population-level data from the national death certificate database of Korea for liver disease and liver cancer showed that the annual number of patients receiving oral antivirals increased from 1,716 to 187,226 from 1999 to 2013. Long-term treatment induced histological improvement, including reversal of cirrhosis and reducing the incidence of HCC [24,25]. The 5-year cumulative incidence of cirrhosis decreased from 23% before the era of antiviral therapy to 5.3% in the era of antiviral therapy, that of hepatic decompensation from 5% to 0.3%, and the incidence of HCC from 3% to 0.8% [26].
There was a dissociation of mortality trends between liver disease and liver cancer despite use of antiviral agents. The crude death rate (CDR) for liver disease decreased from 21.2 to 7.5 per 100,000 persons (64.6% decrease). This reduction is far greater than that reported in a study from Taiwan (8%), also an HBV-endemic area [27]. In contrast, the CDR for liver cancer increased from 20.5% to 22.6% (10.2% increase). The mean age at death from liver disease increased by 8.8 years, significantly greater than the 6.1-year increase for liver cancer and 6.3-year increase for the entire population during the same period. The marked reduction in liver disease mortality due to newborn vaccination and widespread use of antivirals for HBV may have increased the life expectancy and number of patients at risk of developing liver cancer, inadvertently leading to an increased burden of liver cancer in HBV-endemic areas [28,29]. Although the mortality rate of liver disease has decreased, patients with liver cancer have a high mortality rate. This suggests the need for early screening for HCC in patients with HBV infection.
Risk factors that influence the disease course
Both virologic and non-virologic factors influence disease progression and survival in chronic HBV-infected patients. The factors associated with disease activity include HBeAg seropositivity, HBV DNA and HBsAg levels, and HBV genotype. HBV variants such as core promoter or pre-S deletion variants are associated with progressive liver disease and HCC [30,31]. Among these factors, the serum HBV DNA level is the cardinal predictor of disease progression in patients with a high viral load (≥ 2,000 IU/mL), and is a determinant of the risk of progression in those with an HBV DNA level < 2,000 IU/mL [32].
In HBeAg-negative patients with an HBV DNA level < 2,000 IU/mL, the serum HBsAg level is predictive of liver disease progression. An HBsAg level > 1,000 IU/mL is associated with an increased risk of disease progression [33,34]. Among HBeAg-positive patients, a high viral load is generally not predictive of complications, but a moderately high HBV DNA level (> 20,000 IU/mL) and advanced age are closely associated with an increased risk of HCC compared to that of immune‑active phase CHB patients treated with nucleos(t)ide analogues. This suggests the need for earlier antiviral treatment in certain immune-tolerant CHB patients [35].
Spontaneous HBsAg clearance occurs infrequently; the annual incidence is 0.5% to 2.3% [36-38]. The annual HBsAg seroclearance rate is lower in Korea (0.76%) than in other countries. The difference may be due to HBV genotype; the majority of Korean patients are infected with genotype C HBV [39]. After HBsAg seroclearance, the annual rate of HCC development was 0.55% and the associated risk factors were liver cirrhosis, male gender, and age ≥ 45 to 50 years at the time of HBsAg seroclearance [40,41]. Therefore, HCC surveillance is required in patients with these risk factors even after HBsAg seroclearance.
Economic burden of HBV-related disease
In 2005, the societal cost of HBV infection was 1.937 trillion KRW, including direct costs of 474,642 million KRW and indirect costs of 1.463 trillion KRW (Table 2) [42]. This is equivalent to 0.24% of the 2005 Korean gross domestic product (GDP), or 3.98% of Korea’s 2005 national health expenditure (NHE) and 2.01% of annual national health insurance expenditure. However, the economic burden is anticipated to decrease due to vaccination and prevention of perinatal transmission.
Table 2.
Societal costs of hepatitis B virus-related diseases in 2005 (million KRW, %)
| Liver disease | Direct costs | Indirect costs | Total |
|---|---|---|---|
| Chronic hepatitis B | 134,528 | 330,640 | 465,167 (24) |
| Cirrhosis | 112,284 | 421,165 | 433,446 (27.8) |
| Hepatocellular carcinoma | 173,084 | 690,856 | 863,940 (44.6) |
| Liver transplantation | 54,746 | 19,888 | 74,635 (3.9) |
| Total costs | 474,642 | 1,462,549 | 1,937,191 (100) |
Values are presented as number (%). Adapted from Yang et al. [42], with permission from Springer Nature.
KRW, Korea won.
STRATEGIES TO CONTROL HEPATITIS B
Vaccination
The risk of becoming an HBsAg carrier is 90% for perinatal infection, 25% to 30% for infection acquired during the preschool years, and less than 10% for adults [43]. Korea was classified as an area of high endemicity before implementing universal vaccination; in the 1980s, around 11% of neonates in Korea were infected with HBV via vertical transmission [6]. The domestically developed plasma-derived hepatitis B vaccine was approved in Korea in 1983. The first dose of monovalent hepatitis B vaccine is given as soon as possible after birth, preferably within 24 hours, followed by at least two subsequent booster doses at 1 and 6 months. After the introduction of hepatitis B vaccination for all newborns, the coverage rate was 79%; this was further increased to 94% (third dose) through the implementation of the EPI in 1995, higher than the global rate of 84% [4].
Prevention of vertical transmission
The Korea Advisory Committee on Immunization Practices (KACIP) implemented the Hepatitis B Perinatal Transmission Prevention Program (HBPTPP) in July 2002. This program aimed to screen all pregnant women for CHB infection, provide prophylactic treatment (hepatitis B immunoglobulin [HBIG]) to all infants born to HBsAg-positive mothers, and vaccinate all infants against hepatitis B. To ensure compliance among providers and patients in the private sector, the government provided full financial support. A voucher system was implemented to cover the cost of vaccination and to track progress. Voucher information is sent to the Korea CHB database.
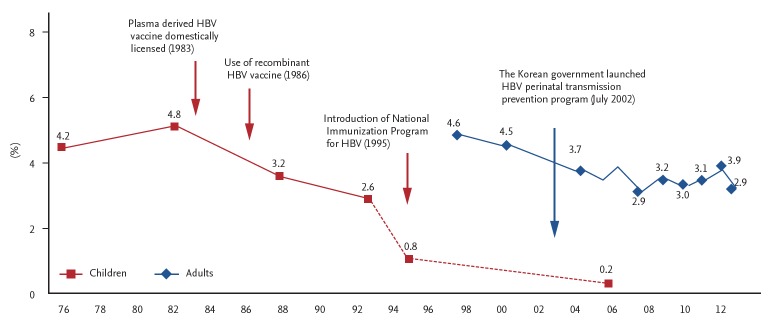
Infants born to HBsAg-positive mothers receive HBIG at birth in addition to vaccination. This has reduced the HBsAg prevalence among blood donors to 0% to 0.4% (Fig. 4), and the third-dose coverage rate among infants is 94% (Table 3). In 2008, the WHO certified that Korea has achieved control of vertical transmission of HBV [44].
Figure 4.
Changes in the prevalence of hepatitis B virus (HBV) carriers after introduction of the national HBV vaccination program. Adapted from Cho et al. [2].
Table 3.
Registered infants born to hepatitis B surface antigen (HBsAg)-positive mothers in the Hepatitis B Perinatal Transmission Prevention Program, July 2002 to June 2012
| Total | 2002 (Jul–) | 2003 | 2004 | 2005 | 2006 | 2007 | 2008 | 2009 | 2010 | 2011 | 2012 (Jan–Jun) | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. of infants at risk of perinatal hepatitis B infection | 153,235 | 7,857 | 16,678 | 16,074 | 14,791 | 15,237 | 15,783 | 14,909 | 14,235 | 15,045 | 15,084 | 7,542 |
| No. of newly registered infants | 147,737 | 5,394 | 14,586 | 15,410 | 14,411 | 15,002 | 16,483 | 15,266 | 14,547 | 14,760 | 14,976 | 6,902 |
| Registered, % | 96.4 | 68.7 | 87.5 | 95.9 | 97.4 | 98.5 | 104.4 | 102.4 | 102.2 | 98.1 | 97.1 | 91.5 |
Estimated number of infants at risk of perinatal hepatitis B infection = number of live births × 0.032 (HBsAg seropositivity of pregnant women). Adapted from Korea Centers for Disease Control & Prevention [1].
Antiviral therapy in pregnant women with high viremia
The HBPTPP was reviewed in 2011 and among 69,999 children that were enrolled in this program with available follow-up serological test results, prophylaxis failure rate was 3.14%. A high viral load in the mother is a major risk factor for post-birth HBV vaccine failure [45]. Immunoprophylaxis may fail in infants born to mothers with an HBV DNA level > 200,000 IU/mL, HBeAg seropositivity, and an HBsAg level < 10,000 IU/mL [46,47]. Recent study from China showed that prophylactic tenofovir in HBsAg-positive mothers with an HBV DNA level > 200,000 IU/mL during the third trimester decreased the rate of mother-to-child transmission [48]. Concerning the safety of tenofovir, no adverse events have been observed in mothers and infants [49]. Our recent meta-analysis also showed that tenofovir prevented vertical transmission and had no significant adverse events [50]. Global guidelines now recommend tenofovir to prevent vertical transmission in the third trimester of pregnancy [16], but health insurance does not cover the cost of antiviral therapy for pregnant women with only a high HBV DNA level. Thus, these data could provide evidence to a policy change for the coverage of antiviral costs in addition to vaccination program.
Catch-up vaccination
In the national immunization guidelines for adults, catch-up hepatitis B vaccinations are recommended for adults without a history of immunization or who had past HBV infection, particularly those with chronic liver diseases, hemodialysis, human immunodeficiency virus infection, or who are frequent recipients of blood products, as well as persons at elevated risk of exposure to HBV (residents and staff of facilities for people with developmental disabilities, prisoners, employees of correctional facilities, people with household contact to someone who is HBsAg-positive, injection drug users, or people with sexually transmitted infections) [45].
Management of HBV infection in immigrant populations
According to monthly statistics published by the Ministry of Justice and Korea Immigration Service, in 2015 there were 1,774,603 foreigners in Korea, more than half of whom were from HBV-endemic areas. Ongoing immigration from countries with high endemicity such as China (918,951, 51.8%), Vietnam (129,423, 7.3%), the Philippines (51,836, 2.9%), and Indonesia (42,520, 2.4%), as well as the increasing number of North Korean defectors (HBV prevalence, 10.9%), represents a public health threat to South Korea and warrants government intervention.
CONCLUSIONS
Hepatitis B vaccination has substantially reduced the prevalence of HBV carriage, and the development of highly effective antivirals has decreased the incidence of liver disease. Advances in prevention and management within three decades have changed the epidemiology of CHB and give cause for optimism that hepatitis B will be eliminated by 2030 followed by reduction in societal burden. However, further effort is required in a new perspective such as prevention of HBV infection in immigration population and coverage of antiviral costs by a national program in pregnant women with high viremia.
Footnotes
No potential conflict of interest relevant to this article was reported.
REFERENCES
- 1.Korea Centers for Disease Control & Prevention . Korea National Health and Nutrition Examination Survey (KNHANES) 2016 [Internet] Cheongju (KR): KCDC; 2016. [cited 2019 Feb 11]. Available from: http://knhanes.cdc.go.kr. [Google Scholar]
- 2.Cho EJ, Kim SE, Suk KT, et al. Current status and strategies for hepatitis B control in Korea. Clin Mol Hepatol. 2017;23:205–211. doi: 10.3350/cmh.2017.0104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kim BH, Park JW. Epidemiology of liver cancer in South Korea. Clin Mol Hepatol. 2018;24:1–9. doi: 10.3350/cmh.2017.0112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.World Health Organization . Global Hepatitis Report 2017. Geneva (CH): WHO; 2017. [Google Scholar]
- 5.World Health Organization . Hepatitis B vaccines: WHO position paper (July 2017) [Internet] Geneva (CH): WHO; c2019. [cited 2019 Feb 11]. Available from: https://www.who.int/immunization/policy/position_papers/hepatitis_b/en/ [DOI] [PubMed] [Google Scholar]
- 6.Park NH, Chung YH, Lee HS. Impacts of vaccination on hepatitis B viral infections in Korea over a 25-year period. Intervirology. 2010;53:20–28. doi: 10.1159/000252780. [DOI] [PubMed] [Google Scholar]
- 7.Lee HS, Byun JH, Kim CY. Etiology and outcome of acute viral hepatitis in Korean adults. J Korean Med Sci. 1990;5:149–154. doi: 10.3346/jkms.1990.5.3.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jeong SH. Current status and vaccine indication for hepatitis A virus infection in Korea. Korean J Gastroenterol. 2008;51:331–337. [PubMed] [Google Scholar]
- 9.Yim HJ, Chang YJ, Byun KS, et al. The changing patterns of acute hepatitis B infection in Korea in the early 2000’s. Korean J Med. 2005;69:601–607. [Google Scholar]
- 10.Choi HJ, Ko SY, Choe WH, et al. Clinical features of acute viral hepatitis B in Korea: a multi-center study. Korean J Hepatol. 2011;17:307–12. doi: 10.3350/kjhep.2011.17.4.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yotsuyanagi H, Yasuda K, Iino S, et al. Persistent viremia after recovery from self-limited acute hepatitis B. Hepatology. 1998;27:1377–1382. doi: 10.1002/hep.510270526. [DOI] [PubMed] [Google Scholar]
- 12.Rehermann B, Ferrari C, Pasquinelli C, Chisari FV. The hepatitis B virus persists for decades after patients’ recovery from acute viral hepatitis despite active maintenance of a cytotoxic T-lymphocyte response. Nat Med. 1996;2:1104–1108. doi: 10.1038/nm1096-1104. [DOI] [PubMed] [Google Scholar]
- 13.Marusawa H, Uemoto S, Hijikata M, et al. Latent hepatitis B virus infection in healthy individuals with antibodies to hepatitis B core antigen. Hepatology. 2000;31:488–495. doi: 10.1002/hep.510310232. [DOI] [PubMed] [Google Scholar]
- 14.Yuki N, Nagaoka T, Yamashiro M, et al. Long-term histologic and virologic outcomes of acute self-limited hepatitis B. Hepatology. 2003;37:1172–1179. doi: 10.1053/jhep.2003.50171. [DOI] [PubMed] [Google Scholar]
- 15.Pattullo V. Prevention of hepatitis B reactivation in the setting of immunosuppression. Clin Mol Hepatol. 2016;22:219–237. doi: 10.3350/cmh.2016.0024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.European Association for the Study of the Liver Electronic address: easloffice@easloffice.eu; European Association for the Study of the Liver. EASL 2017 clinical practice guidelines on the management of hepatitis B virus infection. J Hepatol. 2017;67:370–398. doi: 10.1016/j.jhep.2017.03.021. [DOI] [PubMed] [Google Scholar]
- 17.Korean Association for the Study of the Liver KASL clinical practice guidelines: management of chronic hepatitis B. Clin Mol Hepatol. 2016;22:18–75. doi: 10.3350/cmh.2016.22.1.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Villeneuve JP, Desrochers M, Infante-Rivard C, et al. A long-term follow-up study of asymptomatic hepatitis B surface antigen-positive carriers in Montreal. Gastroenterology. 1994;106:1000–1005. doi: 10.1016/0016-5085(94)90760-9. [DOI] [PubMed] [Google Scholar]
- 19.Fattovich G, Bortolotti F, Donato F. Natural history of chronic hepatitis B: special emphasis on disease progression and prognostic factors. J Hepatol. 2008;48:335–352. doi: 10.1016/j.jhep.2007.11.011. [DOI] [PubMed] [Google Scholar]
- 20.Fattovich G, Giustina G, Schalm SW, et al. Occurrence of hepatocellular carcinoma and decompensation in western European patients with cirrhosis type B. The EUROHEP Study Group on Hepatitis B Virus and Cirrhosis. Hepatology. 1995;21:77–82. doi: 10.1002/hep.1840210114. [DOI] [PubMed] [Google Scholar]
- 21.Liaw YF, Lin DY, Chen TJ, Chu CM. Natural course after the development of cirrhosis in patients with chronic type B hepatitis: a prospective study. Liver. 1989;9:235–241. doi: 10.1111/j.1600-0676.1989.tb00405.x. [DOI] [PubMed] [Google Scholar]
- 22.Yoon SK, Chun HG. Status of hepatocellular carcinoma in South Korea. Chin Clin Oncol. 2013;2:39. doi: 10.3978/j.issn.2304-3865.2013.11.08. [DOI] [PubMed] [Google Scholar]
- 23.Jung KW, Won YJ, Kong HJ, Lee ES, Community of Population-Based Regional Cancer Registries Cancer statistics in Korea: incidence, mortality, survival, and prevalence in 2015. Cancer Res Treat. 2018;50:303–316. doi: 10.4143/crt.2018.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wong GL, Chan HL, Mak CW, et al. Entecavir treatment reduces hepatic events and deaths in chronic hepatitis B patients with liver cirrhosis. Hepatology. 2013;58:1537–1547. doi: 10.1002/hep.26301. [DOI] [PubMed] [Google Scholar]
- 25.Wu CY, Lin JT, Ho HJ, et al. Association of nucleos(t)ide analogue therapy with reduced risk of hepatocellular carcinoma in patients with chronic hepatitis B: a nationwide cohort study. Gastroenterology. 2014;147:143–151. doi: 10.1053/j.gastro.2014.03.048. [DOI] [PubMed] [Google Scholar]
- 26.Kim CY, Kim JW, Lee HS, Yoon YB, Song IS. Natural history and survival rate of chronic liver diseases in Korea: 20 years prospective analysis. Korean J Med. 1994;46:168–180. [Google Scholar]
- 27.Chiang CJ, Yang YW, Chen JD, et al. Significant reduction in end-stage liver diseases burden through the national viral hepatitis therapy program in Taiwan. Hepatology. 2015;61:1154–1162. doi: 10.1002/hep.27630. [DOI] [PubMed] [Google Scholar]
- 28.Choi J, Han S, Kim N, Lim YS. Increasing burden of liver cancer despite extensive use of antiviral agents in a hepatitis B virus-endemic population. Hepatology. 2017;66:1454–1463. doi: 10.1002/hep.29321. [DOI] [PubMed] [Google Scholar]
- 29.Yim SY, Seo YS, Jung CH, et al. The management and prognosis of patients with hepatocellular carcinoma: what has changed in 20 years? Liver Int. 2016;36:445–453. doi: 10.1111/liv.12960. [DOI] [PubMed] [Google Scholar]
- 30.Yim SY, Um SH, Young Jung J, et al. Clinical significance of hepatitis B virus precore and core promoter variants in Korean patients with chronic hepatitis B. J Clin Gastroenterol. 2015;49:61–68. doi: 10.1097/MCG.0000000000000052. [DOI] [PubMed] [Google Scholar]
- 31.Lin CL, Kao JH. New perspectives of biomarkers for the management of chronic hepatitis B. Clin Mol Hepatol. 2016;22:423–431. doi: 10.3350/cmh.2016.0069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lin CL, Tseng TC, Kao JH. What can we learn from hepatitis B virus clinical cohorts? Liver Int. 2015;35 Suppl 1:91–99. doi: 10.1111/liv.12716. [DOI] [PubMed] [Google Scholar]
- 33.Tseng TC, Liu CJ, Yang HC, et al. High levels of hepatitis B surface antigen increase risk of hepatocellular carcinoma in patients with low HBV load. Gastroenterology. 2012;142:1140–1149. doi: 10.1053/j.gastro.2012.02.007. [DOI] [PubMed] [Google Scholar]
- 34.Tseng TC, Liu CJ, Yang HC, et al. Serum hepatitis B surface antigen levels help predict disease progression in patients with low hepatitis B virus loads. Hepatology. 2013;57:441–450. doi: 10.1002/hep.26041. [DOI] [PubMed] [Google Scholar]
- 35.Kim GA, Lim YS, Han S, et al. High risk of hepatocellular carcinoma and death in patients with immune-tolerant-phase chronic hepatitis B. Gut. 2018;67:945–952. doi: 10.1136/gutjnl-2017-314904. [DOI] [PubMed] [Google Scholar]
- 36.Simonetti J, Bulkow L, McMahon BJ, et al. Clearance of hepatitis B surface antigen and risk of hepatocellular carcinoma in a cohort chronically infected with hepatitis B virus. Hepatology. 2010;51:1531–1537. doi: 10.1002/hep.23464. [DOI] [PubMed] [Google Scholar]
- 37.Zhu L, Zhai X, Wang Q, et al. Incidence and determinants of spontaneous hepatitis B surface antigen seroclearance and seroconversion in hepatitis B e antigen-negative chronic infection patients: a population-based prospective cohort. J Viral Hepat. 2018;25:1588–1598. doi: 10.1111/jvh.12978. [DOI] [PubMed] [Google Scholar]
- 38.Ahn SH, Park YN, Park JY, et al. Long-term clinical and histological outcomes in patients with spontaneous hepatitis B surface antigen seroclearance. J Hepatol. 2005;42:188–194. doi: 10.1016/j.jhep.2004.10.026. [DOI] [PubMed] [Google Scholar]
- 39.Park YM, Lee SG. Clinical features of HBsAg seroclearance in hepatitis B virus carriers in South Korea: a retrospective longitudinal study. World J Gastroenterol. 2016;22:9836–9843. doi: 10.3748/wjg.v22.i44.9836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kim GA, Lee HC, Kim MJ, et al. Incidence of hepatocellular carcinoma after HBsAg seroclearance in chronic hepatitis B patients: a need for surveillance. J Hepatol. 2015;62:1092–1099. doi: 10.1016/j.jhep.2014.11.031. [DOI] [PubMed] [Google Scholar]
- 41.Kim JH, Lee YS, Lee HJ, et al. HBsAg seroclearance in chronic hepatitis B: implications for hepatocellular carcinoma. J Clin Gastroenterol. 2011;45:64–68. doi: 10.1097/MCG.0b013e3181dd558c. [DOI] [PubMed] [Google Scholar]
- 42.Yang BM, Kim DJ, Byun KS, Kim HS, Park JW, Shin S. The societal burden of HBV-related disease: South Korea. Dig Dis Sci. 2010;55:784–793. doi: 10.1007/s10620-009-0786-4. [DOI] [PubMed] [Google Scholar]
- 43.Ganem D, Prince AM. Hepatitis B virus infection: natural history and clinical consequences. N Engl J Med. 2004;350:1118–1129. doi: 10.1056/NEJMra031087. [DOI] [PubMed] [Google Scholar]
- 44.Cho HY, Kim CH, Go UY, Lee HJ. Immunization decision-making in the Republic of Korea: the structure and functioning of the Korea Advisory Committee on Immunization Practices. Vaccine. 2010;28 Suppl 1:A91–A95. doi: 10.1016/j.vaccine.2010.02.040. [DOI] [PubMed] [Google Scholar]
- 45.World Health Organization Regional Office for the Western Pacific Meeting report: the third hepatitis B expert resource panel consultation. 2015 Jan 12-13; Seoul, Korea. Available from: http://iris.wpro.who.int/bitstream/handle/10665.1/11153/RS_2015_GE_64_KOR_eng.pdf.
- 46.Brown RS, Jr, McMahon BJ, Lok AS, et al. Antiviral therapy in chronic hepatitis B viral infection during pregnancy: a systematic review and meta-analysis. Hepatology. 2016;63:319–333. doi: 10.1002/hep.28302. [DOI] [PubMed] [Google Scholar]
- 47.Wen WH, Huang CW, Chie WC, et al. Quantitative maternal hepatitis B surface antigen predicts maternally transmitted hepatitis B virus infection. Hepatology. 2016;64:1451–1461. doi: 10.1002/hep.28589. [DOI] [PubMed] [Google Scholar]
- 48.Pan CQ, Duan Z, Dai E, et al. Tenofovir to prevent hepatitis B transmission in mothers with high viral load. N Engl J Med. 2016;374:2324–2334. doi: 10.1056/NEJMoa1508660. [DOI] [PubMed] [Google Scholar]
- 49.Lin Y, Liu Y, Ding G, et al. Efficacy of tenofovir in preventing perinatal transmission of HBV infection in pregnant women with high viral loads. Sci Rep. 2018;8:15514. doi: 10.1038/s41598-018-33833-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hyun MH, Lee YS, Kim JH, et al. Systematic review with meta-analysis: the efficacy and safety of tenofovir to prevent mother-to-child transmission of hepatitis B virus. Aliment Pharmacol Ther. 2017;45:1493–1505. doi: 10.1111/apt.14068. [DOI] [PubMed] [Google Scholar]