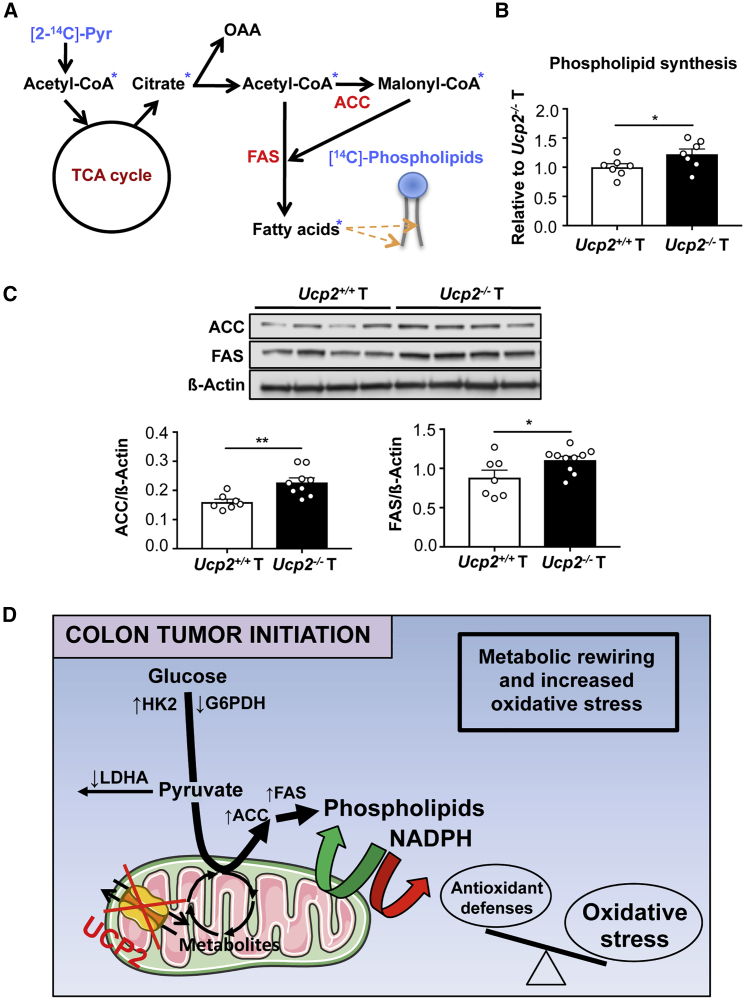
Figure 6.
UCP2 Loss Promotes Phospholipid Synthesis and Increases Expression of Lipogenic Enzymes in Colon Tumors
(A) Scheme showing the incorporation of radioactivity from [2-14C]-pyruvate into phospholipids.
(B) [2-14C]-pyruvate esterification into phospholipids. Data indicate means ± SEMs (n = 7).
(C) Representative immunoblot analysis of ATP citrate lyase (ACLY), ACC, and FAS using whole-cell extracts. Data indicate means ± SEMs (n = 7–10).
(D) Altered metabolic and redox landscape accompanying UCP2 loss favoring colon tumor initiation. Green arrows indicate upregulated reactions, and red arrows indicate downregulated reactions. UCP2 loss in colon tumors redirects glucose metabolism toward phospholipid synthesis. This is achieved by increasing the protein expression of HK2, ACC, and FAS and decreasing the enzyme activity of LDHA and G6PDH. The high demand of NADPH for fatty acid synthesis depletes the levels of this cofactor within Ucp2−/− colon tumors, becoming limiting for the antioxidant defense system. Consequently, the levels of oxidative stress increase in Ucp2−/− colon tumors, impairing the cellular redox balance.