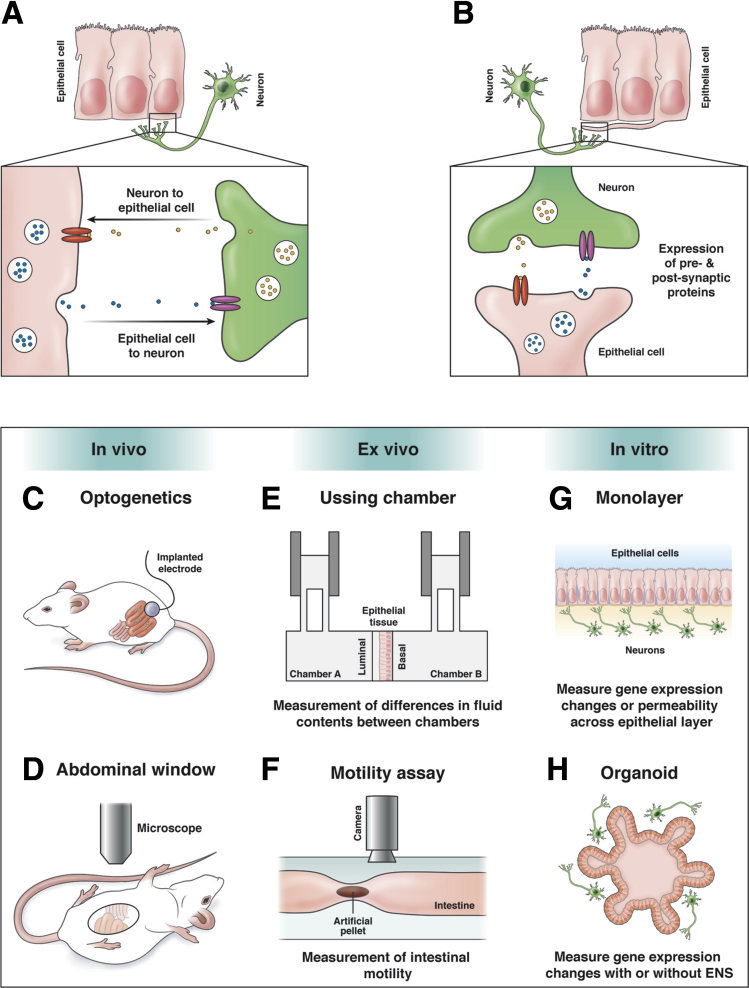
Figure 2.
Mechanisms of epithelial-ENS interactions and current techniques. (A) Paracrine model of neuron-epithelial cell communication. Diffusion of molecules between intestinal epithelial cells and enteric neurons may result in communication in both directions. Release of molecules (yellow or blue circles) from one cell can bind to receptors (red or magenta ovals) on the signal-receiving cell resulting in downstream signaling. (B) Depiction of direct synaptic connections between neurons and epithelial neuropod cells. Expression of synaptic proteins between the neuron and neuropod cell allows for direct communication between the two cells. As with diffusion, expression of hormones or neurotransmitters (yellow or blue circles) from the presynaptic cell binds to receptors (red or magenta ovals) on the opposing postsynaptic cell. Expression of pre- and postsynaptic proteins has been shown with this model. (C–H) Approaches used to study epithelial-ENS interactions using in vitro, ex vivo, or in vivo techniques: (C) optogenetics to manipulate ENS activity in vivo; (D) abdominal window to visualize intestinal activity in vivo in anesthetized animals; and (E) Ussing chamber to study secretion, signaling, or barrier function with ENS cultured on basal side. (The Ussing chamber can be an ex vivo or in vitro technique depending on type of epithelial tissue or cell monolayer used); (F) intestinal motility assay measures propagation of artificial fecal pellet in ex vivo setting; (G) co-culture of monolayers; or (H) organoids allowing for measurement of in vitro gene expression changes or barrier permeability with or without ENS influence on epithelium.