Abstract
BACKGROUND:
Published case series have suggested a potential association between human papillomavirus (HPV) vaccination and primary ovarian insufficiency (POI). We describe POI incidence and estimate POI risk after HPV; tetanus toxoid, reduced diphtheria toxoid, and acellular pertussis, adsorbed (Tdap); inactivated influenza (II); and meningococcal conjugate (MenACWY) vaccination.
METHODS:
We searched Kaiser Permanente Northwest electronic health records for outpatient diagnoses suggestive of POI in female patients aged 11 to 34 years between 2006 and 2014. We reviewed and adjudicated the medical record to confirm diagnoses and estimate symptom onset dates. We excluded cases with known causes and calculated the incidence of idiopathic POI. We estimated risk by calculating hazard ratios and 95% confidence intervals (CIs).
RESULTS:
From a cohort of 199 078 female patients, we identified 120 with diagnoses suggestive of POI. After adjudication and exclusion of 26 POI cases with known causes, we confirmed 46 idiopathic POI cases. POI incidence was low in 11- to 14-year-olds (0.87 per 1 000 000 person-months) and increased with age. One confirmed case patient received the HPV vaccine 23 months before the first clinical evaluation for delayed menarche. The adjusted hazard ratio was 0.30 (95% CI: 0.07–1.36) after HPV, 0.88 (95% CI: 0.37–2.10) after Tdap, 1.42 (95% CI: 0.59–3.41) after II, and 0.94 (95% CI: 0.27–3.23) after MenACWY vaccination.
CONCLUSIONS:
We did not find a statistically significant elevated risk of POI after HPV, Tdap, II, or MenACWY vaccination in this population-based retrospective cohort study. These findings should lessen concern about POI risk after adolescent vaccination.
Human papillomavirus (HPV) is the most common sexually transmitted infection in the United States1 and is associated with several cancers as well as anogenital warts. Since 2006, HPV vaccines have been licensed and recommended for use in adolescent girls by the Advisory Committee on Immunization Practices.2 Rates of HPV vaccination have lagged behind coverage rates for other recommended adolescent vaccinations, such as tetanus toxoid, reduced diphtheria toxoid, and acellular pertussis, adsorbed (Tdap) and meningococcal conjugate (MenACWY). On the basis of national coverage estimates from 2016, 65% of 13- to 17-year old girls received at least 1 HPV vaccination and only 49.5% were up to date with the series, compared with ~88% of adolescents who had received the Tdap vaccine.3 Although authors of large population-based studies have demonstrated the safety of HPV vaccination, parental safety concerns, including potential impacts on future fertility, are often cited as 1 reason for lower HPV coverage.4–8
Concern about infertility after HPV vaccination developed after case series were published describing the onset of primary ovarian insufficiency (POI), also known as premature ovarian failure or premature menopause, within 12 months after vaccination in 6 young women from 13 to 21 years of age.9,10 POI is characterized by either the dysfunction or depletion of ovarian follicles, menopausal symptoms (eg, amenorrhea or hot flashes), or reduced fertility. In girls <20 years of age, POI is uncommon with an estimated prevalence of 1 case per 10 000.11 Chromosomal abnormalities, including Turner syndrome and Fragile X syndrome, as well as the gonadotoxic treatment of cancer (chemotherapy or radiation) are known etiologies for POI; however, most POI is idiopathic but may be associated with underlying autoimmune (eg, rheumatoid arthritis or systemic lupus erythematosus), metabolic (eg, galactosemia), or infectious disease (eg, mumps).11
Reports of POI after HPV vaccination have garnered national media attention and have circulated widely on social media and other Internet sites,12–15 but to our knowledge, no population-based studies of POI after HPV vaccination have been conducted to date. The published case series must be interpreted with caution because the authors of the series included small samples of young women presenting for care at selected clinical sites, relied on self-reported vaccine exposures, and lacked controls. We conducted a retrospective cohort study to (1) identify and describe characteristics of idiopathic POI diagnosed in female patients 11 to 34 years of age, (2) describe the prevalence and age-specific incidence of POI, and (3) estimate the risk of idiopathic POI in female patients after HPV vaccination or other recommended adolescent vaccinations (Tdap, MenACWY, and inactivated influenza [II]).
METHODS
The Vaccine Safety Datalink (VSD) is a collaborative project involving 8 integrated health care delivery systems and the Centers for Disease Control and Prevention. The current VSD study was conducted at a single site, Kaiser Permanente Northwest (KPNW), serving a population of ~570 000 members in Oregon and Washington. The study protocol, data collection tools, and study procedures were approved by the KPNW Institutional Review Board.
Study Period and Population
We identified all female patients 11 to 34 years of age with at least 30 days of health plan enrollment at KPNW from August 1, 2006, to December 31, 2014. This study period was selected to maximize the potential number of POI cases in the period after HPV vaccine licensure and recommendation. We also wanted to focus on female patients who were age-eligible for HPV vaccination and to allow for the follow-up of women who were vaccinated at 26 years of age. Cohort participants were managed until health plan disenrollment, the participant’s 35th birthday, or the end of the study period, whichever occurred first.
Case Ascertainment
We identified presumptive cases of POI among young women in the cohort by searching electronic health record (EHR) databases for outpatient encounters coded with International Classification of Diseases, Ninth Revision (ICD-9) codes for premature menopause (256.31), other ovarian failure (256.39), other ovarian dysfunction (256.8), and unspecified ovarian dysfunction (256.9). We then manually reviewed the medical record of the presumptive cases to collect data on diagnostic and other laboratory testing, symptom onset, and other POI risk factors. The study abstraction form was developed in partnership with subject matter experts from the Centers for Disease Control and Prevention and 2 KPNW obstetrics and gynecology providers.
Each presumptive case was abstracted by at least 1 study investigator (MLH, SAI, and ALN). Presumptive cases that were clearly miscoded or ruled out diagnoses, or cases for whom adequate medical records were not available, were excluded from additional review. Remaining presumptive cases were then abstracted again independently by a second investigator. Any discrepant information obtained by the 2 independent abstractions was discussed and resolved by the investigator team. As part of the medical record review, we either recorded the date of POI symptom onset when noted or estimated the onset date on the basis of other information documented in the record (eg, age at onset or date of last menses) or the earliest encounter for delayed menarche, amenorrhea, oligomenorrhea, or infertility evaluation.
After medical record review, we excluded cases of POI with a known cause, including those with surgical menopause (ie, removal of the ovaries), those with a genetic condition, such as Fragile X Syndrome, Turner syndrome, or another X-linked chromosomal disorder, and those who had received either chemotherapy or radiotherapy for cancer. The remaining idiopathic presumptive POI cases were reviewed and adjudicated by an obstetrics and gynecology nurse practitioner to confirm case status.
The American College of Obstetricians and Gynecologists (ACOG) guidelines for the diagnosis of POI16 include the presence of menstrual irregularity for at least 3 months and elevated follicle-stimulating hormone (FSH) in the postmenopausal range and low estradiol levels on 2 separate occasions. Other clinical tests used to establish or refine the diagnosis include karyotyping, adrenal antibody titers, pelvic ultrasonography, and antimullerian hormone levels. Many presumptive cases could not be classified with the ACOG definition because the diagnostic tests were not consistently ordered by the treating providers. The clinical adjudicator was instructed to classify presumptive cases as probable POI if there was strong evidence to support a diagnosis of POI and/or most or all of the ACOG definition was met; possible POI was classified if there was some evidence to support a diagnosis of POI, but the ACOG definition was not met; and not POI was classified if the case clearly did not meet the ACOG definition.
Descriptive Analysis
We calculated the proportion of the presumptive cases identified by each ICD-9 code that met the case definitions of probable and possible POI as defined above after medical record review and adjudication. We also described demographic characteristics, diagnostic testing patterns and results, symptom onset, and time to diagnosis among confirmed (probable and possible) idiopathic cases.
POI Incidence
We calculated the prevalence of idiopathic diagnosed POI by dividing the number of confirmed (probable and possible) cases identified during the study period by the number of eligible cohort members; POI incidence was calculated by using eligible person-months as the denominator. Person-months were calculated based on the beginning and end of the study period and each individual’s health plan enrollment dates as described above. We calculated age-stratified (11–14, 15–18, 19–22, 23–26, 27–30, and 31–34 years) rates on the basis of the date of POI diagnosis and calculated 95% confidence intervals (CIs) for these rates on the basis of the Poisson distribution.
Risk Estimation
We used time-dependent Cox proportional hazards modeling to estimate the hazard ratios (HRs) and 95% CIs associated with HPV, Tdap, MenACWY, or II exposures independently. Because we observed long intervals between symptom onset and POI diagnosis, we limited the cases included in the models to those with symptom onset after August 1, 2006 (ie, the date of HPV vaccine availability at KPNW). To capture data on additional adolescent vaccines that were licensed before HPV, we identified all vaccine exposures in the cohort occurring 1 year before (August 1, 2005) and during the study period. Vaccinations were identified by using both the KPNW EHR databases and the Oregon and Washington state immunization information systems to increase the capture of vaccinations received outside of the KPNW delivery system.
To calculate person-time in the Cox models, we managed subjects until the symptom onset dates for cases, and the end of the study period, the 35th birthday, or last health plan disenrollment during the study period for noncases. We defined vaccine exposure as the first dose received of a specific vaccine from 1 year before the start of the observation period through the end of follow-up. In the absence of any data to suggest a biologically plausible exposure window, we considered any person-time after a specific vaccine exposure to be exposed person-time. We therefore did not specifically model multiple doses of a vaccine or a combination of multiple vaccines. We estimated both crude and age-adjusted HRs for each vaccine and described the timing of vaccine exposures related to POI symptom onset. On the basis of post hoc power calculations, we estimated the smallest significant HRs we could detect with 80% power, and α = .05 were 3.23 for HPV, 2.94 for Tdap, 2.94 for II, and 3.45 for MenACWY exposures.
RESULTS
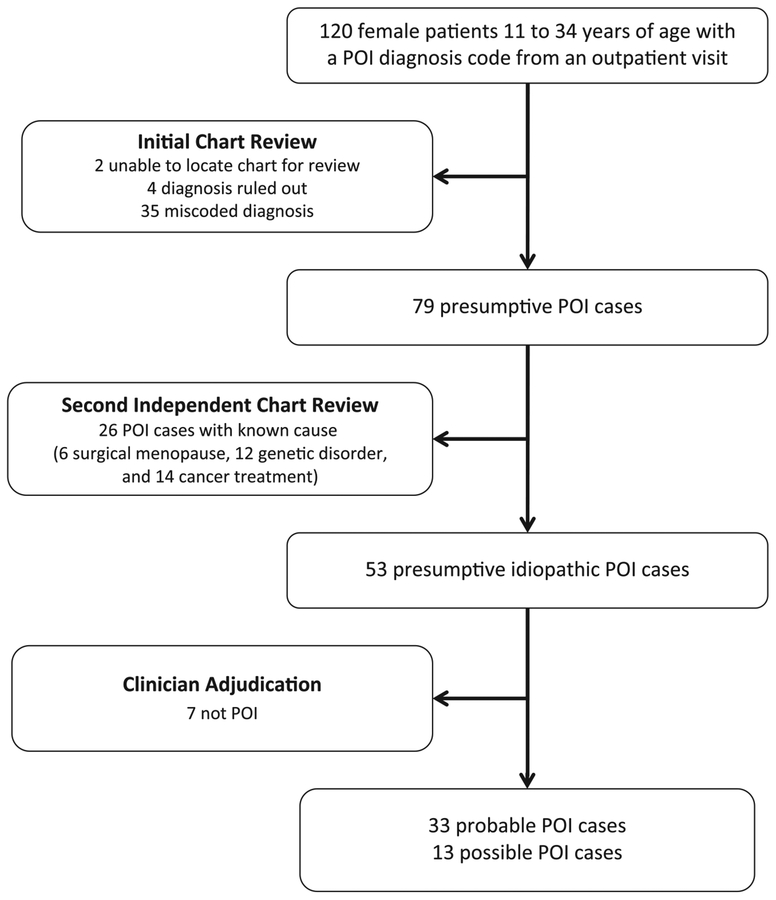
During the study period (August 1, 2006, to December 31, 2014), 199 078 female patients 11 to 34 years of age had at least 30 days of health plan enrollment in KPNW; 119 078 received Tdap, 84 783 received II, 58 871 received HPV, and 46 231 received MenACWY vaccination. From this cohort, we identified 120 patients with an outpatient diagnosis of premature menopause, ovarian failure, or ovarian dysfunction (Fig 1). We excluded 41 presumptive cases after the initial chart review; 35 patients had a specific diagnosis other than POI (miscoded POI diagnoses), 4 patients were ruled out POI diagnoses, and 2 patients had inadequate medical records available for review. After the second round of chart review, we excluded 26 POI cases with a known cause, including the following: 6 patients with surgical menopause, 12 patients with a genetic disorder, and 14 patients treated for cancer (note categories are not mutually exclusive). The clinical adjudicator classified 7 presumptive cases as not POI, leaving 46 confirmed, idiopathic POI cases (33 probable and 13 possible).
FIGURE 1.
Ascertainment and adjudication of POI cases, KPNW, August 2006 to December 2014.
Two ICD-9 codes, 256.8 (other ovarian dysfunction) and 256.9 (unspecified ovarian dysfunction), yielded no medical record–confirmed POI cases. Of the 28 presumptive cases diagnosed with ICD-9 code 256.31 (premature menopause), 50% were classified as possible or probable idiopathic POI after full review and adjudication. Similarly, we classified 43% of the 76 presumptive cases diagnosed with ICD-9 256.39 (other ovarian failure) as possible or probable idiopathic POI.
More than one-half of the confirmed POI cases were patients who were diagnosed at ≥27 years of age, and only 1 patient was diagnosed at <15 years of age (Table 1). Most patients were white (61%) and non-Hispanic (67%) individuals. Six patients (13%) presented with primary amenorrhea, and 8 patients (17%) had a comorbid autoimmune disease diagnosis. Symptom onset could not always be estimated if there was insufficient documentation in the medical record or if the patient presented with primary amenorrhea. Excluding these cases, the median time from symptom onset to diagnosis was ~3 years (range: 75 days–16 years, data not shown).
TABLE 1.
Characteristics of Confirmed Idiopathic POI Cases, KPNW, August 2006 to December 2014
| Characteristic | N (%) |
|---|---|
| Age at diagnosis, y | |
| 11–14 | 1 (2) |
| 15–18 | 5 (11) |
| 19–22 | 4 (9) |
| 23–26 | 7 (15) |
| 27–30 | 13 (28) |
| 31–34 | 16 (35) |
| Age at estimated symptom onset, y | |
| 11–14 | 6 (13) |
| 15–18 | 9 (20) |
| 19–22 | 5 (11) |
| 23–26 | 11 (24) |
| 27–30 | 10 (28) |
| 31–34 | 5 (11) |
| Race | |
| White | 28 (61) |
| Multiracial or people of color | 5 (11) |
| Unknown | 13 (28) |
| Ethnicity | |
| Latina and/or Hispanic | 7 (15) |
| Non-Latina or non-Hispanic | 31 (67) |
| Unknown | 8 (17) |
| Autoimmune comorbid diagnosis | 8 (17) |
| Primary amenorrhea | 6 (13) |
| Family history of POI | 4 (9) |
| Family history of infertility | 4 (9) |
Most patients had at least 1 FSH (94%) or estradiol (78%) test in their medical record, but multiple tests were less common, especially for estradiol (Table 2). Only 9 (20%) confirmed cases met the strict ACOG diagnostic criteria for POI. Testing for antiadrenal and thyroperoxidase antibodies was uncommon, as were abnormal antimullerian hormone levels.
TABLE 2.
Diagnostic Testing for POI, KPNW, August 2006 to December 2014
| Test | N (%) |
|---|---|
| ACOG tests | |
| FSH tested | 43 (94) |
| FSH tested on at least 2 separate occasions | 33 (72) |
| FSH was abnormala on at least 2 separate occasions | 33 (72) |
| Estradiol tested | 36 (78) |
| Estradiol tested on at least 2 separate occasions | 16 (35) |
| Estradiol was abnormala on at least 2 separate occasions | 9 (20) |
| Met ACOG POI case definitionb | 9 (20) |
| Other diagnostic tests | |
| Abnormal antimullerian hormone | 4 (9) |
| Antiadrenal antibodies | 0 (0) |
| Thyroperoxidase antibodies | 4 (9) |
Abnormal is defined as being in the postmenopausal range by using reference values documented in the medical record. Reference values varied by patient age, reference laboratory, and the timing of collection relative to menstrual cycle and hormonal therapy use.
ACOG POI case definition requires the presence of menstrual irregularity for at least 3 mo, elevated FSH in the postmenopausal range, and low estradiol levels on 2 separate occasions.
The prevalence of idiopathic POI in the study period was 2.31 per 10 000 female patients. The incidence of diagnosed POI increased with age, from a low of 0.87 per 1 000 000 person-months in 11- to 14-year-olds to a peak of 12.85 per 1 000 000 person-months in 30- to 34-year-olds (Table 3).
TABLE 3.
Age-Specific Incidence of Diagnosed POI, KPNW, August 2006 to December 2014
| Age at Initial Diagnosis, y | Cases | Person-Mo | Incidence per 1 000 000 Person-Mo (95% CI) |
|---|---|---|---|
| 11–14 | 1 | 1 151 805 | 0.87 (0.12–6.16) |
| 15–18 | 5 | 1 226 602 | 4.08 (1.70–9.79) |
| 19–22 | 4 | 1 109 535 | 3.61 (1.35–9.61) |
| 23–26 | 7 | 1 059 109 | 6.61 (3.15–13.86) |
| 27–30 | 13 | 1 151 201 | 11.29 (6.56–19.45) |
| 31–34 | 16 | 1 245 185 | 12.85 (7.87–20.97) |
Of the confirmed cases, 18 (39%) had symptom onset before August 1, 2006, and were excluded from Cox models. Of the remaining 28 cases, only 1 was vaccinated against HPV before symptom onset; this 16-year-old girl received her third dose of HPV vaccine ~23 months before the estimated symptom onset. This patient presented with primary amenorrhea, so the symptom onset date was estimated as the earliest documented encounter for clinical evaluation of delayed menarche; POI onset likely occurred before this estimated date. The age-adjusted HR of POI after HPV vaccination was 0.30 (95% CI: 0.07–1.36) (Table 4).
TABLE 4.
POI Incidence in Vaccinated and Unvaccinated Young Women and Associated HRs With 95% CIs
| Cases Vaccinated Before Symptom Onset | Unexposed Cases | Unadjusted HR (95% CI) | Age-Adjusted HR (95% CI) | |
|---|---|---|---|---|
| HPV | 1 | 27 | 0.27 (0.06–1.16) | 0.30 (0.07–1.36) |
| Tdap | 6 | 22 | 0.78 (0.34–1.84) | 0.88 (0.37–2.10) |
| MenACWY | 3 | 25 | 0.70 (0.24–2.03) | 0.94 (0.27–3.23) |
| II | 11 | 17 | 1.35 (0.57–3.22) | 1.42 (0.59–3.41) |
Six confirmed patients with POI were vaccinated with Tdap (5–40 months before symptom onset), 11 patients were vaccinated with II (2–91 months before symptom onset), and 3 patients were vaccinated with MenACWY (2–27 months before symptom onset). The age-adjusted HR of POI was 0.88 (95% CI: 0.37–2.10) with Tdap, 1.42 (95% CI: 0.59–3.41) with II, and 0.94 (95% CI: 0.27–3.23) with MenACWY vaccination (Table 4).
DISCUSSION
To our knowledge, this is the first population-based study in which POI is evaluated as a possible vaccine adverse event. We did not find an elevated risk of POI after HPV vaccination, nor did we find any elevated risk after Tdap, II, or MenACWY vaccination. We observed only 1 patient with POI who possibly had symptom onset after HPV vaccination from a population of 58 871 young women who received the HPV vaccine during the study period. If POI is triggered by HPV or other adolescent vaccine exposure, we would have expected to see elevated incidence in the younger women who were most likely to be vaccinated, but instead we observed higher incidence in older women (>26 years of age), which is consistent with 1 other population-based study of POI prevalence.11 With our study, we overcame some of the limitations of the previously published case reports describing POI after HPV vaccination, including small sample sizes and lack of population-based controls, and this should provide additional reassurance about the safety of HPV vaccination with respect to POI risk. However, it should be noted that this study was underpowered to detect small increases in POI risk associated with vaccination.
Studying POI as a vaccine adverse event is challenging for many reasons. First, the time from symptom onset to diagnosis with POI may be variable or long.17 We observed that the median time from symptom onset to POI diagnosis was 3 years in this cohort, and the authors of another study reported that 25% of patients required >5 years from onset to diagnosis.17 We were able to capture cases that were diagnosed in our health plan, but we may have misclassified some true cases of POI who were symptomatic but did not have a long enough follow-up time to reach a diagnosis. However, because 81% of our cohort was managed for >24 months (mean follow-up time = 5.14 years), we consider that this potential for misclassification was minimal.
Secondly, diagnoses of POI are difficult to accurately identify. We found that using the ACOG diagnostic definition was difficult to implement in observational studies derived from EHR data because patients often did not undergo all of the diagnostic testing required to meet the definition.16 In our health care system, we observed that patients were likely to receive 1 FSH and 1 estradiol test but not the multiple tests required for positive diagnosis based on the ACOG definition; review and adjudication by a women’s health clinician was therefore needed to establish case status. Additionally, we excluded some POI cases with a known cause, such as certain genetic disorders like Turner syndrome, and focused on idiopathic POI that might be triggered by vaccination. Other case definitions for epidemiological studies have varied, and many researchers did not exclude POI with a known cause, making comparison of rates and estimation of risk difficult across studies.18–20
Another challenge of this study, and all observational studies of POI, is adequately capturing hormonal contraceptive use, which may mask the symptoms and onset of POI and can result in misclassification.21, 22 Many of the patients we observed in this cohort were women in their late 20s who stopped using contraceptives because they were attempting to conceive, and subsequently presented for diagnostic evaluation of amenorrhea and infertility. We were not able to adjust for contraceptive use because these data are not routinely included in the VSD data files. Additionally, the capture of contraceptives for young women may not be complete in health plan databases if young women are receiving and filling prescriptions at outside pharmacies and clinics. We believe that the potential misclassification of case status in women using hormonal contraceptives would be nondifferential because authors of previous studies have not found that hormonal conceptive use is more prevalent among vaccinated young women compared with unvaccinated women.23,24
If vaccination is associated with POI onset, the exposure would most likely induce POI through an autoimmune pathway.25,26 There are no mechanistic studies available to suggest what a biologically plausible exposure window might be, and so we examined any exposure before symptom onset. Using this long exposure window has the potential to mask a true acute risk of POI after vaccination, but limiting our study to shorter windows might miss an association that requires a longer latent period.
All vaccinated patients were exposed at least 2 months before symptom onset, and many were vaccinated years before onset. The 1 patient exposed to HPV who was observed was vaccinated nearly 2 years before the estimated symptom onset date, which is not consistent with the authors of case reports who observed onset within 12 months of vaccination.9,10 Although we did not formally test, we did not observe any temporal clustering of vaccine exposures among patients.
CONCLUSIONS
Concern about a potential association between HPV vaccination and POI generated by earlier published case series and media attention may negatively impact decision-making about HPV vaccine acceptance. We found no evidence of increased risk of POI after HPV vaccination, or other routine adolescent vaccination exposures, in this population-based retrospective cohort study with nearly 200 000 young women. Despite the challenges and limitations discussed above, we believe this study should lessen concern surrounding potential impact on fertility from HPV or other adolescent vaccination.
WHAT’S KNOWN ON THIS SUBJECT:
Authors of published case series have suggested an association between human papillomavirus vaccination and primary ovarian insufficiency, but no population-based epidemiological studies have been reported.
WHAT THIS STUDY ADDS:
No significant elevated risk of primary ovarian insufficiency after adolescent vaccination was observed in this population-based retrospective cohort study of nearly 200 000 young women. These findings should lessen concern about potential impact on fertility from adolescent vaccination.
ACKNOWLEDGMENT
We thank Kate Beadle, nurse practitioner, for her assistance with the adjudication of medical records.
FINANCIAL DISCLOSURE: Dr Naleway has received research funding from Pfizer, MedImmune AstraZeneca, and Merck for unrelated studies. Ms Irving has received research support from MedImmune AstraZeneca for an unrelated study. Dr Henninger has received research funding from Pfizer for an unrelated study; the other authors have indicated they have no financial relationships relevant to this article to disclose.
FUNDING: Supported by the US Centers for Disease Control and Prevention (task order 200-2012-53584-0006). The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
ABBREVIATIONS
- ACOG
American College of Obstetricians and Gynecologists
- CI
confidence interval
- EHR
electronic health record
- FSH
follicle-stimulating hormone
- HPV
human papillomavirus
- HR
hazard ratio
- ICD-9
International Classification of Diseases, Ninth Revision
- II
inactivated influenza
- KPNW
Kaiser Permanente Northwest
- MenACWY
meningococcal conjugate
- POI
primary ovarian insufficiency
- Tdap
tetanus toxoid, reduced diphtheria toxoid, and acellular pertussis, adsorbed
- VSD
Vaccine Safety Datalink
Footnotes
POTENTIAL CONFLICT OF INTEREST: Dr Naleway has received research funding from Pfizer, MedImmune AstraZeneca, and Merck for unrelated studies. Ms Irving has received research support from MedImmune AstraZeneca for an unrelated study. Dr Henninger has received research funding from Pfizer for an unrelated study; the other authors have indicated they have no potential conflicts of interest to disclose.
REFERENCES
- 1.Satterwhite CL, Torrone E, Meites E, et al. Sexually transmitted infections among US women and men: prevalence and incidence estimates, 2008. Sex Transm Dis. 2013;40(3):187–193 [DOI] [PubMed] [Google Scholar]
- 2.Petrosky E, Bocchini JA Jr, Hariri S, et al. ; Centers for Disease Control and Prevention. Use of 9-valent human papillomavirus (HPV) vaccine: updated HPV vaccination recommendations of the advisory committee on immunization practices. MMWR Morb Mortal Wkly Rep. 2015;64(11):300–304 [PMC free article] [PubMed] [Google Scholar]
- 3.Walker TY, Elam-Evans LD, Singleton JA, et al. National, regional, state, and selected local area vaccination coverage among adolescents aged 13–17 years - United States, 2016. MMWR Morb Mortal Wkly Rep. 2017;66(33):874–882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bingham A, Drake JK, LaMontagne DS. Sociocultural issues in the introduction of human papillomavirus vaccine in low-resource settings. Arch Pediatr Adolesc Med. 2009;163(5):455–461 [DOI] [PubMed] [Google Scholar]
- 5.Verhoeven V, Baay MF, Baay PE, Lardon F, Van Royen P, Vermorken JB. Everything you always wanted to know about HPV (but could not ask your doctor). Patient Educ Couns. 2010;81(1):101–105 [DOI] [PubMed] [Google Scholar]
- 6.Young A. HPV vaccine acceptance among women in the Asian Pacific: a systematic review of the literature. Asian Pac J Cancer Prev. 2010;11(3):641–649 [PubMed] [Google Scholar]
- 7.Cover JK, Nghi NQ, LaMontagne DS, Huyen DT, Hien NT, Nga le T. Acceptance patterns and decision-making for human papillomavirus vaccination among parents in Vietnam: an in-depth qualitative study post-vaccination. BMC Public Health. 2012;12:629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Glenn BA, Tsui J, Coronado GD, et al. Understanding HPV vaccination among Latino adolescent girls in three U.S. regions. J Immigr Minor Health. 2015;17(1):96–103 [DOI] [PubMed] [Google Scholar]
- 9.Little DT, Ward HR. Premature ovarian failure 3 years after menarche in a 16-year-old girl following human papillomavirus vaccination. BMJ Case Rep. 2012;2012:bcr2012006 879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Colafrancesco S, Perricone C, Tomljenovic L, Shoenfeld Y. Human papilloma virus vaccine and primary ovarian failure: another facet of the autoimmune/inflammatory syndrome induced by adjuvants. Am J Reprod Immunol. 2013;70(4):309–316 [DOI] [PubMed] [Google Scholar]
- 11.Cox L, Liu JH. Primary ovarian insufficiency: an update. Int J Womens Health. 2014;6:235–243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sears A. “They’ve been robbed of their womanhood:” two sisters face one life-changing diagnosis. 2014. Available at: http://fox6now.com/2014/11/13/theyve-been-robbed-of-their-womanhood-two-sisters-face-one-life-changing-diagnosis/. Accessed June 22, 2018
- 13.Wetzstein C. HPV vaccine cited in infertility case: Wis. sisters say drug led to ovarian failure by age 16. The Washington Times; November 11, 2013. Available at: https://www.washingtontimes.com/news/2013/nov/11/hpv-vaccine-cited-in-infertility-case/. Accessed June 22, 2018 [Google Scholar]
- 14.Favoloro M. Young women claim HPV vaccine left them infertile. The Legal Examiner. December 5, 2013. Available at: http://norfolk.legalexaminer.com/fda-prescription-drugs/young-women-claim-hpv-vaccine-left-them-infertile/. Accessed June 22, 2018 [Google Scholar]
- 15.Jonathan; SaneVax, Inc. Infertility concern with gardasil HPV vaccine: does the HPV vaccine LITERALLY mean “one less”? 2011. Available at: http://sanevax.org/infertility-concern-with-gardasil-hpv-vaccine/. Accessed June 22, 2018
- 16.The American College of Obstetricians and Gynecologists. Committee opinion no. 605: primary ovarian insufficiency in adolescents and young women. Obstet Gynecol. 2014;124(1):193–197 [DOI] [PubMed] [Google Scholar]
- 17.Alzubaidi NH, Chapin HL, Vanderhoof VH, Calis KA, Nelson LM. Meeting the needs of young women with secondary amenorrhea and spontaneous premature ovarian failure. Obstet Gynecol. 2002;99(5, pt 1):720–725 [DOI] [PubMed] [Google Scholar]
- 18.Coulam CB, Adamson SC, Annegers JF. Incidence of premature ovarian failure. Obstet Gynecol. 1986;67(4):604–606 [PubMed] [Google Scholar]
- 19.Sadrzadeh S, Painter RC, van Kasteren YM, Braat DD, Lambalk CB. Premature ovarian insufficiency and perinatal parameters: a retrospective case-control study. Maturitas. 2017;96:72–76 [DOI] [PubMed] [Google Scholar]
- 20.Haller-Kikkatalo K, Uibo R, Kurg A, Salumets A. The prevalence and phenotypic characteristics of spontaneous premature ovarian failure: a general population registry-based study. Hum Reprod. 2015;30(5):1229–1238 [DOI] [PubMed] [Google Scholar]
- 21.Welt CK. Clinical manifestations and evaluation of spontaneous primary ovarian insufficiency (premature ovarian failure). 2017. Available at: https://www.uptodate.com/contents/clinical-manifestations-and-diagnosis-of-spontaneous-primary-ovarianin-sufficiency-premature-ovarian-failure. Accessed April 27, 2017 [Google Scholar]
- 22.Little D. Quadrivalent human papillomavirus vaccine and the young ovary: review of safety research following two case series of premature ovarian insufficiency. J Immunol Infect Dis. 2017;4(1):101 [Google Scholar]
- 23.Jena AB, Goldman DP, Seabury SA. Incidence of sexually transmitted infections after human papillomavirus vaccination among adolescent females. JAMA Intern Med. 2015;175(4):617–623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bednarczyk RA. Human papillomavirus vaccine and sexual activity: how do we best address parent and physician concerns? JAMA Intern Med. 2015;175(4):624–625 [DOI] [PubMed] [Google Scholar]
- 25.Pellegrino P, Carnovale C, Pozzi M, et al. On the relationship between human papilloma virus vaccine and autoimmune diseases. Autoimmun Rev. 2014;13(7):736–741 [DOI] [PubMed] [Google Scholar]
- 26.Gruber N, Shoenfeld Y. A link between human papilloma virus vaccination and primary ovarian insufficiency: current analysis. Curr Opin Obstet Gynecol. 2015;27(4):265–270 [DOI] [PubMed] [Google Scholar]