Figure 1.
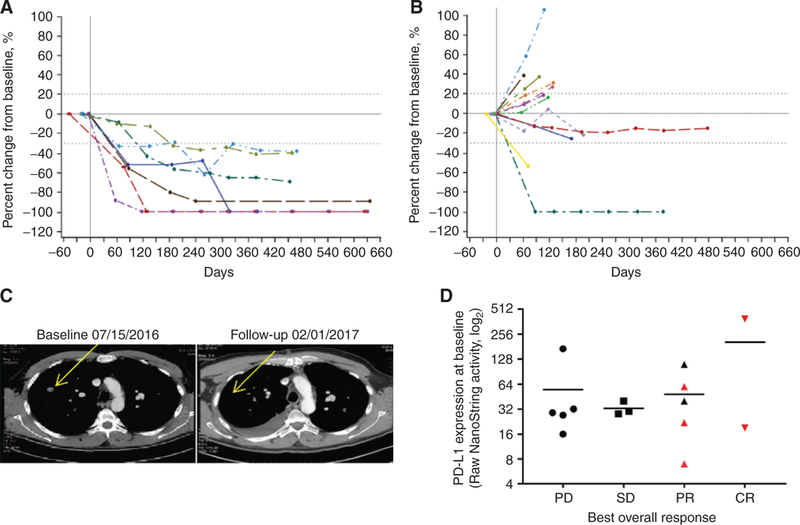
Responses to the combination of SD-101 and pembrolizumab. Percent change from baseline over time in tumor burden in all target lesions in patients naïve to anti-PD-1/PD-L1 therapy (A) and in patients with prior anti-PD-1/PD-L1 therapy (B). Dashed lines denote cutoffs for PD (+20%) or response (−30%) according to RECIST v1.1. C, Response to therapy in a noninjected lesion is shown in a CT scan from a 61-year-old male patient with stage IV disease at baseline. The patient had not received anti-PD-1/PD-L1 therapy prior to enrollment and had a PR to SD-101/pembrolizumab therapy. D, PD-L1 RNA expression profiling using the NanoString PanCancer Immune Profiling Panel at baseline by best overall response. Red symbols indicate patients who did not have prior anti-PD-1 therapy.
