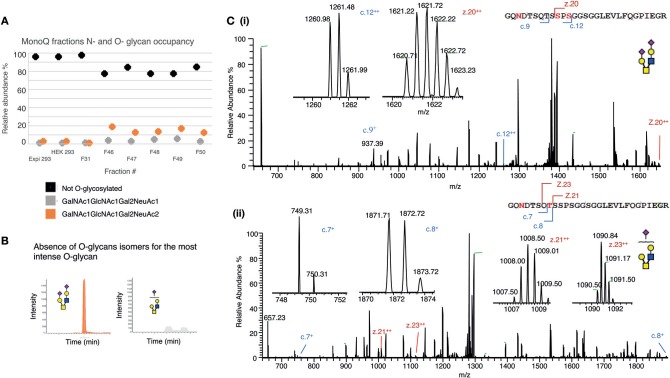
Figure 6.
Mapping the O-glycosylation of recombinant human CD52. (A) N- and O-glycan occupancy of CD52 I, CD52 II, and selected MonoQ fractions (F31 and F46–F51) measured at the protein level after de-N-glycosylation. (B) PGC resolution of O-glycosylated isomers from active fractions m/z 665.22− (GalNAc1GlcNAc1Gal2NeuAc2) and m/z 1040.41− (GalNAc1GlcNAc1Gal2NeuAc1). (C) EThcD-MS/MS based site localization analysis showing the peptide backbone fragments and the ions diagnostic of the amino acid site for both aforementioned O-glycans. Fragmentation to c and z ions are shown that indicate that (i) di-sialylated O-glycans were conjugated to Ser12, and possibly Ser10, whereas (ii) the mono-sialylated O-glycans are found on Thr8.