Figure 1: Origin and Function of Cardiac Macrophages in Mice and Humans.
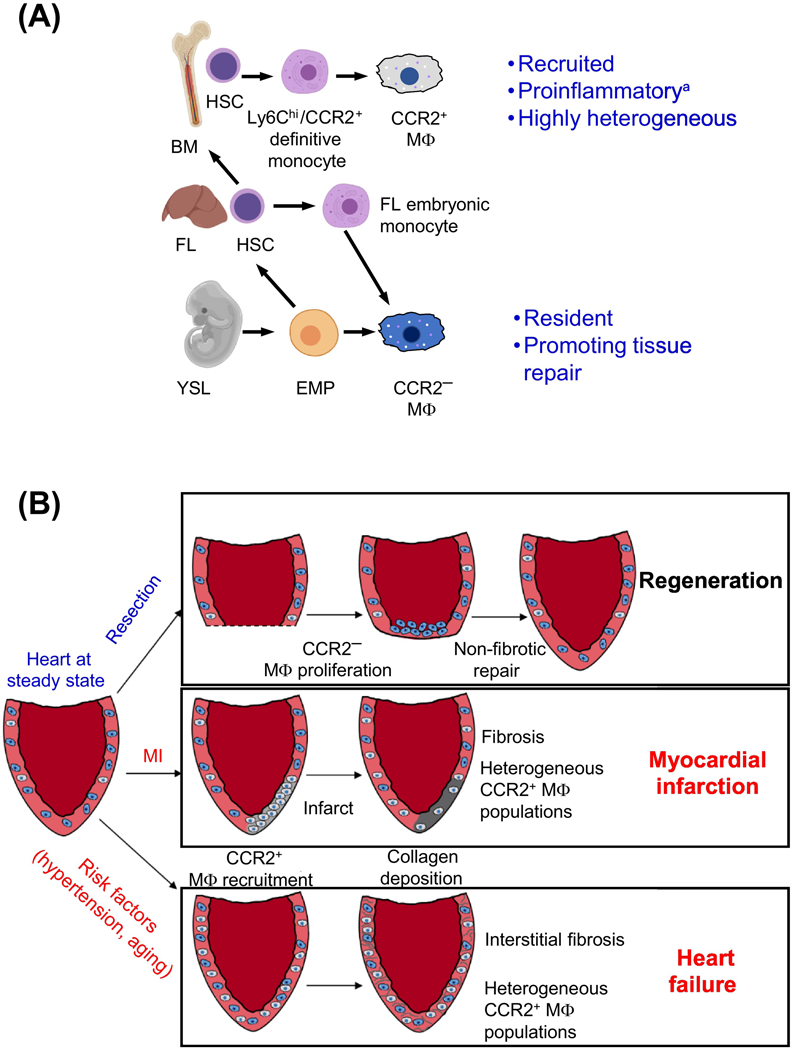
(a) CCR2+ macrophages are derived from recruited Ly6chi/CCR2+ monocytes that develop from definitive hematopoietic stem cells with origins in the fetal liver and bone marrow. CCR2- macrophages differentiate from erythro-myeloid progenitors (EMPs) that are specified in the yolk sac as well as hematopoietic stem cells from the fetal liver. Fetal liver monocytes, which are derived from a population of yolk syncytial layer EMPs, remain a poorly understood population. (b) Models of macrophage roles in heart regeneration and disease are shown. In hearts with regenerative capacity, such as neonatal mice and lower vertebrates, injury/resection results in local proliferation of CCR2- macrophages at the site of injury, promoting non-fibrotic repair. Following myocardial infarction in adult murine hearts without regenerative capacity, resident CCR2- macrophages die and monocyte derived macrophages, which retain CCR2 expression, are recruited to the infarct where they promote inflammation and stimulate collagen deposition resulting in localized fibrosis at the site of injury. In the case of heart failure, CCR2- macrophages are recruited to the failing heart but are not localized to a single site, resulting in formation of interstitial fibrosis that stiffens the ventricular wall, decreasing cardiac function. Radiolabeled probes have also demonstrated that CCR2+ cells are recruited to acute MI sites and failing hearts in humans. *While recruited monocyte derived macrophages represent predominantly pro-inflammatory macrophages, a smaller population of recruited macrophages demonstrate wound-healing properties in contrast to the majority of the recruited population. FL – Fetal Liver, BM – Bone Marrow, HSC – Hematopoietic Stem Cell, YSL – Yolk Syncytial Layer, EMP – Erythro-Myeloid Progenitor
