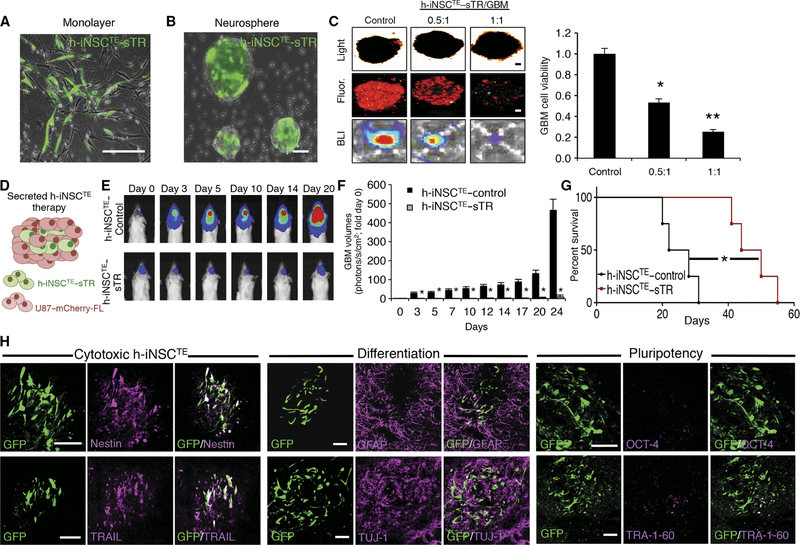
Fig. 5. h-iNSCTE–mediated TRAIL therapy for solid GBM.
(A and B) Representative fluorescence photomicrographs depict h-iNSCTE engineered to secrete the proapoptotic agent TRAIL and grown in a monolayer (A) or as floating neurospheres (B). Expression of the internal ribosomal entry sites (IRES)–GFP element present in the construct is shown in green. (C) Images and summary data of 3D suspension cultures showing the viability of mCherry+ human U87 GBM spheroids (red) mixed with therapeutic h-iNSCTE–sTR or control cells at a ratio of 0.5:1 or 1:1. GBM spheroid viability was determined by BLI 48 hours after treatment. **P = 0.0169, *P = 0.038 by ANOVA. (D) h-iNSCTE–sTR therapy for solid GBM was performed by xenografting a mixture of h-iNSCTE–sTR and U87 GBM cells into the brain parenchyma of severe combined immunodeficient mice. (E and F) Representative BLI (E) and summary data (F) demonstrating the inhibition of solid U87 GBM progression by h-iNSCTE–sTR therapy compared to control-treated mice. *P = 0.0044 by repeated-measures ANOVA. (G) Kaplan-Meier survival curves demonstrating the extension in survival in h-iNSCTE–sTR–treated animals compared to h-iNSCTE–control. *P = 0.0067 by log-rank test. (H) Representative images demonstrating the expression of cytotoxic, differentiation, and pluripotency markers in h-iNSCTE–sTR after therapy. A subset of animals were sacrificed 14 days after therapy; brain sections were stained with antibodies against nestin, TRAIL, GFAP, TUJ-1, OCT-4, or TRA-1–60; and the colocalization between staining (magenta) and GFP+ h-iNSCTE–sTR (green) was visualized. Data in (C) are means ± SEM of three independent experiments performed in triplicate. Data in (F) are means ± SEM. Scale bars, 100 μm.