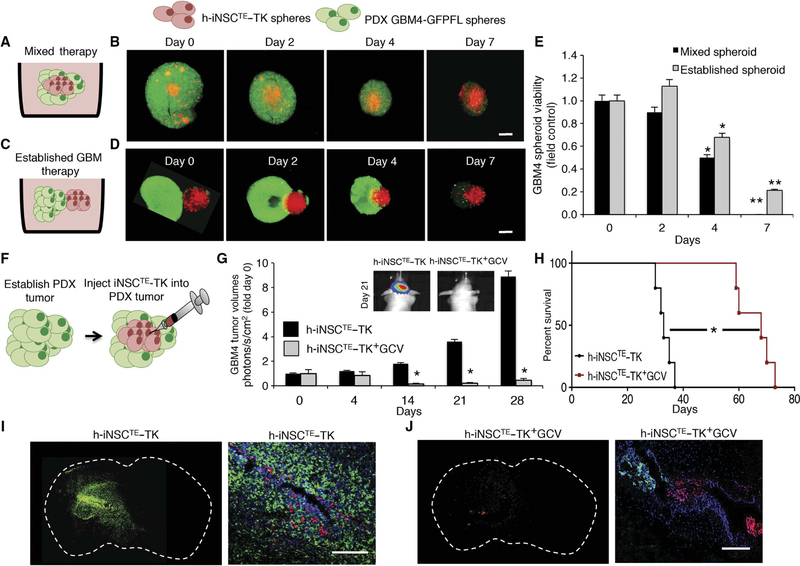
Fig. 6. h-iNSCTE prodrug/enzyme therapy for human patient–derived GBMs.
(A to D) The antitumor effects of h-iNSCTE–TK therapy were determined in two different 3D culture models. h-iNSCTE–TK (red) were either mixed GFP+ GBM4 patient-derived GBM cells (A and B) or seeded adjacent to established GBM4 spheroids (C and D), and GCV was added to initiate tumor killing. Serial fluorescence images showed the time-dependent decrease in GBM4 spheroid volume by h-iNSCTE–TK+GCV therapy. (E) Summary graph demonstrating the reduction in GBM4 spheroid volume over 7 days by h-iNSCTE–TK+GCV therapy either mixed or seeded adjacent to established spheroids. **P = 0.0099, *P = 0.048 by ANOVA. (F to H) h-iNSCTE–TK therapy was assessed in vivo by injecting h-iNSCTE–TK cells into GBM4 tumors established 10 days earlier in the brains of mice (F). Serial BLI showed that the progression of GBM4 tumors was inhibited by h-iNSCTE–TK+GCV therapy (G). *P = 0.0046 by repeated-measures ANOVA. (H) Kaplan-Meier survival curves demonstrate the survival of mice bearing GBM4 tumors treated with h-iNSCTE–TK+GCV therapy or control h-iNSCTE. *P = 0.0018 by log-rank test. (I and J) Representative whole-brain and high-magnification images showing cell nuclei (blue), GBM4 (green), and h-iNSCTE–TK (red) distribution 21 days after delivering h-iNSCTE–control (I) or h-iNSCTE–TK (J) into established GBM4 tumors. A large GBM4 tumor was present in the control h-iNSCTE–TK animals, and only a small GBM4 focus was detected in mice treated with h-iNSCTE–TK+GCV. Data in (E) are means ± SEM of three independent experiments performed in triplicate. Data in (G) are means ± SEM. Scale bars, 400 mm (B and D) and 200 μm (I and J).