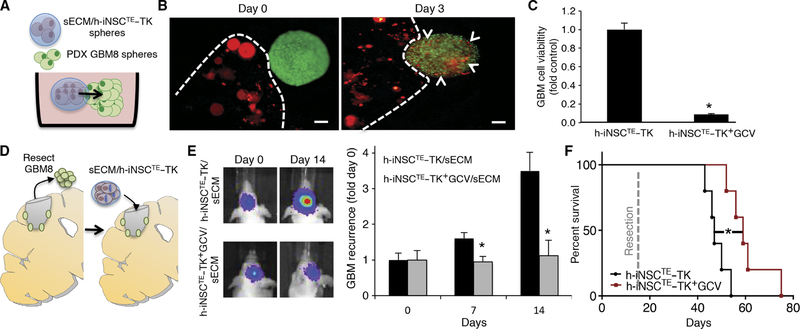
Fig. 7. Intracavity h-iNSCTE–TK therapy for surgically resected diffuse GBMs.
(A to C) 3D suspension cultures were used to assess the migration and antitumor efficacy of sECM-encapsulated h-iNSCTE–TK against patient-derived GBM8 spheroids (A). h-iNSCTE–TK (red) encapsulated in sECM were found to migrate from the matrix and populate GBM8 spheroids 3 days after seeding (B). Summary data demonstrate that h-iNSCTE–TK (red) encapsulated in sECM reduce the volume of GBM8 spheroids (green) after GCV treatment compared to spheroids treated without GCV (C). *P = 0.0016 by Student’s t test. (D to F) To mimic clinical h-iNSCTE therapy for surgically resected GBM, h-iNSCTE–TK were encapsulated in sECM and transplanted into the surgical cavity after resection of diffuse patient-derived GBM8 tumors expressing GFP-FL (D). Representative images and summary data for serial imaging demonstrating the inhibition of tumor recurrence after intracavity h-iNSCTE–TK therapy for postoperative minimal GBM8 tumors (E). *P = 0.0072 by repeated-measures ANOVA. Kaplan-Meier survival curves of mice that underwent surgical resection of diffuse patient-derived GBM8 tumor cells and were treated with control h-iNSCTE or h-iNSCTE–TK encapsulated in sECM and transplanted into the surgical cavity (F). *P = 0.0064 by log-rank test. Data in (C) are means ± SEM of three independent experiments performed in triplicate. Data in (E) are means ± SEM. Scale bars, 200 μm.