Abstract
Background
Although delirium is typically an acute reversible cognitive impairment, its presence is associated with devastating impact on both short‐term and long‐term outcomes for critically ill patients. Advances in our understanding of the negative impact of delirium on patient outcomes have prompted trials evaluating multiple pharmacological interventions. However, considerable uncertainty surrounds the relative benefits and safety of available pharmacological interventions for this population.
Objectives
Primary objective
1. To assess the effects of pharmacological interventions for treatment of delirium on duration of delirium in critically ill adults with confirmed or documented high risk of delirium
Secondary objectives
To assess the following:
1. effects of pharmacological interventions on delirium‐free and coma‐free days; days with coma; delirium relapse; duration of mechanical ventilation; intensive care unit (ICU) and hospital length of stay; mortality; and long‐term outcomes (e.g. cognitive; discharge disposition; health‐related quality of life); and
2. the safety of such treatments for critically ill adult patients.
Search methods
We searched the following databases from their inception date to 21 March 2019: Ovid MEDLINE®, Ovid MEDLINE® In‐Process & Other Non‐Indexed Citations, Embase Classic+Embase, and PsycINFO using the Ovid platform. We also searched the Cochrane Library on Wiley, the International Prospective Register of Systematic Reviews (PROSPERO) (http://www.crd.york.ac.uk/PROSPERO/), the Cumulative Index to Nursing and Allied Health Literature (CINAHL), and Web of Science. We performed a grey literature search of relevant databases and websites using the resources listed in Grey Matters developed by the Canadian Agency for Drugs and Technologies in Health (CADTH). We also searched trial registries and abstracts from annual scientific critical care and delirium society meetings.
Selection criteria
We sought randomized controlled trials (RCTs), including quasi‐RCTs, of any pharmacological (drug) for treatment of delirium in critically ill adults. The drug intervention was to be compared to another active drug treatment, placebo, or a non‐pharmacological intervention (e.g. mobilization). We did not apply any restrictions in terms of drug class, dose, route of administration, or duration of delirium or drug exposure. We defined critically ill patients as those treated in an ICU of any specialty (e.g. burn, cardiac, medical, surgical, trauma) or high‐dependency unit.
Data collection and analysis
Two review authors independently identified studies from the search results; four review authors (in pairs) performed data extraction and assessed risk of bias independently. We performed data synthesis through pairwise meta‐analysis and network meta‐analysis (NMA). Our hypothetical network structure was designed to be analysed at the drug class level and illustrated a network diagram of 'nodes' (i.e. drug classes) and 'edges' (i.e. comparisons between different drug classes from existing trials), thus describing a treatment network of all possible comparisons between drug classes. We assessed the quality of the body of evidence according to GRADE, as very low, low, moderate, or high.
Main results
We screened 7674 citations, from which 14 trials with 1844 participants met our inclusion criteria. Ten RCTs were placebo‐controlled, and four reported comparisons of different drugs. Drugs examined in these trials were the following: antipsychotics (n = 10), alpha2 agonists (n = 3; all dexmedetomidine), statins (n = 2), opioids (n = 1; morphine), serotonin antagonists (n = 1; ondansetron), and cholinesterase (CHE) inhibitors (n = 1; rivastigmine). Only one of these trials consistently used non‐pharmacological interventions that are known to improve patient outcomes in both intervention and control groups.
Eleven studies (n = 1153 participants) contributed to analysis of the primary outcome. Results of the NMA showed that the intervention with the smallest ratio of means (RoM) (i.e. most preferred) compared with placebo was the alpha2 agonist dexmedetomidine (0.58; 95% credible interval (CrI) 0.26 to 1.27; surface under the cumulative ranking curve (SUCRA) 0.895; moderate‐quality evidence). In order of descending SUCRA values (best to worst), the next best interventions were atypical antipsychotics (RoM 0.80, 95% CrI 0.50 to 1.11; SUCRA 0.738; moderate‐quality evidence), opioids (RoM 0.88, 95% CrI 0.37 to 2.01; SUCRA 0.578; very‐low quality evidence), and typical antipsychotics (RoM 0.96, 95% CrI 0.64 to1.36; SUCRA 0.468; high‐quality evidence).
The NMAs of multiple secondary outcomes revealed that only the alpha2 agonist dexmedetomidine was associated with a shorter duration of mechanical ventilation (RoM 0.55, 95% CrI 0.34 to 0.89; moderate‐quality evidence), and the CHE inhibitor rivastigmine was associated with a longer ICU stay (RoM 2.19, 95% CrI 1.47 to 3.27; moderate‐quality evidence). Adverse events often were not reported in these trials or, when reported, were rare; pair‐wise analysis of QTc prolongation in seven studies did not show significant differences between antipsychotics, ondansetron, dexmedetomidine, and placebo.
Authors' conclusions
We identified trials of varying quality that examined six different drug classes for treatment of delirium in critically ill adults. We found evidence that the alpha2 agonist dexmedetomidine may shorten delirium duration, although this small effect (compared with placebo) was seen in pairwise analyses based on a single study and was not seen in the NMA results. Alpha2 agonists also ranked best for duration of mechanical ventilation and length of ICU stay, whereas the CHE inhibitor rivastigmine was associated with longer ICU stay. We found no evidence of a difference between placebo and any drug in terms of delirium‐free and coma‐free days, days with coma, physical restraint use, length of stay, long‐term cognitive outcomes, or mortality. No studies reported delirium relapse, resolution of symptoms, or quality of life. The ten ongoing studies and the six studies awaiting classification that we identified, once published and assessed, may alter the conclusions of the review.
Plain language summary
Medicines to treat delirium in critically ill adult patients
Review question
We reviewed the evidence from randomized controlled trials for the benefits and safety of all prescription medicines used to treat critically ill adult patients with delirium in the intensive care units (ICUs) of hospitals.
Background
Delirium is commonly associated with surgery, infection, or critical illness. It is experienced as new‐onset, generally short‐term inability to think clearly. Patients with delirium shift between periods of clear thinking and periods of agitation and/or great sleepiness and confusion. Lack of sleep, pain, a noisy environment, physical restraint, and the use of sedatives and strong analgesics are some of the contributing factors. Delirium affects both immediate and longer‐term health outcomes of critically ill patients as it can increase the length of time a breathing machine is required, time spent in the ICU and in hospital, and the chance of functional weakening and death. The odds of a poor outcome with delirium are increased with frail patients and those of advanced age and already present cognitive difficulties. Frequently, delirious ICU patients are given medicines to help treat symptoms such as agitation.
Study characteristics
This review is current to 21 March 2019. We found 14 randomized controlled studies that enrolled a total of 1844 adult participants. Six different classes of medicines were tested. These were antipsychotic drugs used as tranquillizers in ten studies; the sedative alpha2 agonist dexmedetomidine in three studies; statins that reduce cholesterol in two studies; opioids as part of pain management in one study; serotonin antagonists for nausea and vomiting in one study; and cholinesterase inhibitors, which are medicines for Alzheimer's disease, in one study. Ten studies compared medicine to placebo ‐ an inactive medicine also known as a sugar pill; four studies compared different drugs. Eleven studies with 1153 participants reported on the main outcome of this review ‐ duration of delirium.
Key findings
When drug classes were directly compared with placebo, only the alpha2 agonist dexmedetomidine was found to reduce the duration of delirium, and the cholinesterase inhibitor rivastigmine was found to prolong the duration of delirium. Each of these results is based on findings from a single small study. The other drugs when compared to placebo did not change delirium duration. The Review authors used the statistical method of network meta‐analysis to compare the six different drug classes. Dexmedetomidine was ranked most effective in reducing delirium duration, followed by atypical antipsychotics. However, network meta‐analysis of delirium duration failed to rule out the possibility of no difference for all six drug classes compared to placebo. Using this method, we did not find that any drug improved the duration of coma, length of stay, long‐term cognitive outcomes, or death. The alpha2 agonist dexmedetomidine shortened time spent on a breathing machine. Adverse events often were not reported in these trials or were rare when reported. An analysis of reported events showed that events were similar to those reported with placebo. We found 10 ongoing studies and six studies awaiting classification that, once published and assessed, may change the conclusions of this review.
Quality of the evidence
Most of the included studies were small but of good design. Nine of the 14 studies were considered to have low risk of bias.
Summary of findings
Summary of findings for the main comparison. Duration of delirium.
| Outcome: duration of delirium | ||||||
|
Patient or population: critically ill adults with confirmed or at high risk of delirium
Settings: intensive care units in Australia and New Zealand, Canada, Egypt, Netherlands, Turkey, USA, UK
Intervention: any pharmacological intervention Control: placebo or active comparator | ||||||
| Comparisons | Illustrative comparative risks* (95% CrI) |
Ratio of means (RoM) based on log RoM estimates from meta‐analysis (IV, random, 95% CI) |
Number of participants (studies) | Quality of the evidence (GRADE) based on NMA | NMA results (assuming consistency equations) | |
| Assumed risk | Corresponding risk based on NMA estimates | |||||
| Placebo/Comparator | Intervention drug | |||||
| Typical antipsychotic vs placebo | Median duration of delirium: 3 to 5 days for placebo | 3.86 days of delirium (95% CrI 2.57 to 5.46) corresponding to 4 days in the placebo group | RoM: exp(0.02) = 1.02 (95% CI 0.91 to 1.14); log RoM: 0.02 (‐0.09 to 0.13); I² = 0% | 608 (4 studies) | ⊕⊕⊕⊕ High | RoM (95% CrI): 0.96 (0.64 to 1.36), SUCRA = 0.468, mean Pr(best) = 0.010, mean rank = 4.19 |
| Atypical antipsychotic vs placebo | Median duration of delirium: 3 to 5 days for placebo | 3.22 days of delirium (95% CrI 2.01 to 4.43) corresponding to 4 days in the placebo group | RoM: exp(‐0.31) = 0.73 (95% CI 0.49 to 1.11); log RoM: ‐0.31 (‐0.71 to 0.10); I² = 82% | 500 (4 studies) | ⊕⊕⊕⊝ Moderatea | RoM (95% CrI): 0.80 (0.50 to 1.11), SUCRA = 0.738, mean Pr(best) = 0.114, mean rank = 2.57 |
|
Statin (HMG‐CoA) vs placebo |
Mean duration of delirium: 6.8 to 8.68 days for placebo | 4.20 days of delirium (95% CrI 2.44 to 7.09) corresponding to 4 days in the placebo group | RoM: exp(0.07) = 1.07 (95% CI 0.91 to 1.25); log RoM: 0.07 (‐0.09 to 0.22); I² = 0% | 414 (2 studies) | ⊕⊕⊕⊝ Moderateb | RoM (95% CrI): 1.05 (0.61 to 1.77), SUCRA = 0.365, mean Pr(best) = 0.023, mean rank = 4.81 |
|
Alpha2 agonist vs placebo |
Median duration of delirium: 2.583 days for placebo | 2.31 days of delirium (95% CrI 1.06 to 5.06) corresponding to 4 days in the placebo group | RoM: exp(‐0.55) = 0.58 (95% CI 0.43 to 0.79); log RoM: ‐0.55 (‐0.85 to ‐0.24); I² not applicable | 71 (1 study) | ⊕⊕⊕⊝ Moderateb | RoM (95% CrI): 0.58 (0.26 to 1.27), SUCRA = 0.895, mean Pr(best) = 0.717, mean rank = 1.63 |
|
Cholinesterase inhibitor vs placebo |
Median duration of delirium: 3 days for placebo | 7.37 days of delirium (95% CrI 3.26 to 16.38) corresponding to 4 days in the placebo group | RoM: exp(0.61) = 1.84 (95% CI 1.25 to 2.69); log RoM: 0.61 (0.22 to 0.99); I² not applicable | 104 (1 study) | ⊕⊕⊕⊝ Moderateb | RoM (95% CrI): 1.84 (0.82 to 4.10), SUCRA = 0.054, mean Pr(best) = 0.006, mean rank = 6.68 |
|
Opioid vs placebo |
No study reported this comparison | 3.53 days of delirium (95% CrI 1.46 to 8.05) corresponding to 4 days in the placebo group |
Pairwise meta‐analysis not performed | 0 (0 studies) | ⊕⊕⊝⊝ Very lowb,c | RoM (95% CrI): 0.88 (0.37 to 2.01), SUCRA = 0.578, mean Pr(best) = 0.129, mean rank = 3.53 |
| *The basis for the assumed risk (e.g. the median control group risk across studies). The corresponding risk (and its 95% CrI) is calculated as the assumed risk multiplied by the ratio of means (and its 95% CrI) based on NMA. Abbreviations: CI: confidence interval; CrI: credible interval; HMG‐CoA: 5‐hydroxy‐3‐methylglutaryl‐coenzyme A reductase inhibitor; NMA: network meta‐analysis; Pr(best): probability(best); RoM: ratio of means; SUCRA: surface under the cumulative ranking curve. | ||||||
| GRADE Working Group grades of evidence. High quality: further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: we are very uncertain about the estimate. | ||||||
aDowngraded one level for heterogeneity (I² > 75% considered as large heterogeneity). bDowngraded one level for imprecision (wide credible interval). cDowngraded two levels for only indirect evidence available and risk of bias of a single trial informing opioid vs typical antipsychotic.
Summary of findings 2. Days with coma.
| Outcome: days with coma | ||||||
|
Patient or population: critically ill adult with confirmed or at high risk of delirium
Settings: intensive care units in Australia and New Zealand, Canada, Egypt, Netherlands, Turkey, USA, UK
Intervention: any pharmacological intervention Control: placebo or active comparator | ||||||
| Comparisons | Illustrative comparative risks* (95% CI) |
Ratio of means (RoM) based on log RoM estimates from meta‐analysis (IV, random, 95% CI) |
Number of participants (studies) | Quality of the evidence (GRADE) based on NMA | NMA results (assuming consistency equations) | |
| Assumed risk | Corresponding risk based on NMA estimates | |||||
| Placebo/Comparator | Intervention drug | |||||
| Typical antipsychotic vs placebo | Median number of days with coma: 1 to 2 days for placebo | 1.53 days with coma (95% CrI 0.86 to 2.57) corresponding to 2 days in the placebo group | RoM: exp(‐0.29) = 0.75 (95% CI 0.49 to 1.13); log RoM: ‐0.29 (‐0.71 to 0.12); I² = 74% | 588 (3 studies) | ⊕⊕⊝⊝ Lowa,b | RoM (95% CrI): 0.77 (0.43 to 1.29), SUCRA = 0.820, mean Pr(best) = 0.620, mean rank = 1.54 |
|
Atypical antipsychotic vs placebo |
Median number of days with coma: 1 to 2 days for placebo | 1.88 days with coma (95% CrI 0.96 to 3.43) corresponding to 2 days in the placebo group | RoM: exp(0.06) = 1.06 (95% CI 0.88 to 1.30); log RoM: 0.06 (‐0.13 to 0.26); I² = 0% | 440 (2 studies) | ⊕⊕⊕⊝ Moderateb | RoM (95% CrI): 0.94 (0.48 to 1.72), SUCRA = 0.422, mean Pr(best) = 0.132, mean rank = 2.73 |
|
Statin (HMG‐CoA) vs placebo |
Mean number of days with coma: 1.1 to 4.2 days for placebo | 1.84 days with coma (95% CrI 0.98 to 3.59) corresponding to 2 days in the placebo group | RoM: exp(‐0.10) = 0.90 (95% CI 0.73 to 1.12); log RoM: ‐0.10 (‐0.32 to 0.11); I² = 0% | 414 (2 studies) | ⊕⊕⊕⊝ Moderateb | RoM (95% CrI): 0.92 (0.49 to 1.80), SUCRA = 0.481, mean Pr(best) = 0.222, mean rank = 2.56 |
| *The basis for the assumed risk (e.g. the median control group risk across studies). The corresponding risk (and its 95% CrI) is calculated as the assumed risk multiples the ratio of means (and its 95% CrI) based on NMA. CI: confidence interval; CrI: credible interval; HMG‐CoA: 5‐hydroxy‐3‐methylglutaryl‐coenzyme A reductase inhibitor; NMA: network meta‐analysis; Pr(best): probability(best); RoM: ratio of means; SUCRA: surface under the cumulative ranking curve. | ||||||
| GRADE Working Group grades of evidence. High quality: further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: we are very uncertain about the estimate. | ||||||
aDowngraded one level for heterogeneity (I² of 50% to 75%, > 75% considered as medium and large heterogeneity). bDowngraded one level for imprecision (wide credible interval).
Summary of findings 3. Duration of mechanical ventilation.
| Outcome: duration of mechanical ventilation | ||||||
|
Patient or population: critically ill adult with confirmed or at high risk of delirium
Settings: intensive care units in Australia and New Zealand, Canada, Egypt, Netherlands, Turkey, USA, UK
Intervention: any pharmacological intervention Control: placebo or active comparator | ||||||
| Comparisons | Illustrative comparative risks* (95% CI) |
Ratio of means (RoM) based on log RoM estimates from meta‐analysis (IV, random, 95% CI) |
Number of participants (studies) | Quality of the evidence (GRADE) based on NMA | NMA results (assuming consistency equations) | |
| Assumed risk | Corresponding risk based on NMA estimates | |||||
| Placebo/Comparator | Intervention drug | |||||
| Typical antipsychotics vs placebo | Median duration of mechanical ventilation: 3 to 5 days for placebo | 3.71 days of mechanical ventilation (95% CrI 2.89 to 4.94) corresponding to 4 days in the placebo group | RoM: exp(‐0.08) = 0.92 (95% CI 0.79 to 1.06); log RoM: ‐0.08 (‐0.23 to 0.06); I² = 0% | 515 (3 studies) | ⊕⊕⊕⊝ Moderatea | RoM (95% CrI): 0.93 (0.72 to 1.24), SUCRA = 0.576, mean Pr(best) = 0.009, mean rank = 3.12 |
| Atypical antipsychotics vs placebo | Median duration of mechanical ventilation: 3 to 11 days for placebo | 3.91 days of mechanical ventilation (95% CrI 2.85 to 5.10) corresponding to 4 days in the placebo group | RoM: exp(‐0.02) = 0.98 (95% CI 0.84 to 1.34); log RoM: ‐0.02 (‐0.17 to 0.14); I² = 0% | 476 (3 studies) | ⊕⊕⊕⊝ Moderatea | RoM (95% CrI): 0.98 (0.71 to 1.28), SUCRA = 0.440, mean Pr(best) = 0.012, mean rank = 3.80 |
| Statin (HMG‐CoA) vs placebo | Mean duration of mechanical ventilation: 11 days for placebo | 4.38 days of mechanical ventilation (95% CrI 2.82 to 6.77) corresponding to 4 days in the placebo group | RoM: exp(0.09) = 1.09 (95% CI 0.90 to 1.34); log RoM: 0.09 (‐0.11 to 0.29); I² not applicable | 272 (1 study) | ⊕⊕⊕⊝ Moderatea | RoM (95% CrI): 1.10 (0.71 to 1.69), SUCRA = 0.223, mean Pr(best) = 0.014, mean rank = 4.88 |
| Alpha2 agonist vs placebo | Median duration of mechanical ventilation: 1.846 days for placebo | 2.21 days of mechanical ventilation (95% CrI 1.36 to 3.58) corresponding to 4 days in the placebo group | RoM: exp(‐0.59) = 0.55 (95% CI 0.41 to 0.75); log RoM: ‐0.59 (‐0.89 to ‐0.29); I² not applicable | 71 (1 study) | ⊕⊕⊕⊝ Moderatea | RoM (95% CrI): 0.55 (0.34 to 0.89), SUCRA = 0.974, mean Pr(best) = 0.931, mean rank = 1.13 |
| Opioid vs placebo | No study reported this comparison | 3.96 days of mechanical ventilation (95% CrI 2.32 to 7.02) corresponding to 4 days in the opioid group | Pairwise meta‐analysis not performed | 0 (0 studies) |
⊕⊕⊝⊝ Very lowa,b | RoM (95% CrI): 0.99 (0.58 to 1.76), SUCRA = 0.410, mean Pr(best) = 0.033, mean rank = 3.95 |
| *The basis for the assumed risk (e.g. the median control group risk across studies). The corresponding risk (and its 95% CrI) is calculated as the assumed risk multiples the ratio of means (and its 95% CrI) based on NMA. CI: confidence interval; CrI: credible interval; HMG‐CoA: 5‐hydroxy‐3‐methylglutaryl‐coenzyme A reductase inhibitor; NMA: network meta‐analysis; Pr(best): probability(best); RoM: ratio of means; SUCRA: surface under the cumulative ranking curve. | ||||||
| GRADE Working Group grades of evidence. High quality: further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: we are very uncertain about the estimate. | ||||||
aDowngraded one level for imprecision (wide credible interval). bDowngraded two levels for only indirect evidence available and risk of bias of a single trial informing opioid vs typical antipsychotic.
Summary of findings 4. Length of ICU stay.
| Outcome: length of ICU stay | ||||||
|
Patient or population: critically ill adult with confirmed or at high risk of delirium
Settings: intensive care units in Australia and New Zealand, Canada, Egypt, Netherlands, Turkey, USA, UK
Intervention: any pharmacological intervention Control: placebo or active comparator | ||||||
| Comparisons | Illustrative comparative risks* (95% CI) |
Ratio of means (RoM) based on log RoM estimates from meta‐analysis (IV, random, 95% CI) |
Number of participants (studies) | Quality of the evidence (GRADE) based on NMA | NMA results (assuming consistency equations) | |
| Assumed risk | Corresponding risk based on NMA estimates | |||||
| Placebo/Comparator | Intervention drug | |||||
| Typical antipsychotic vs placebo | Median length of ICU stay: 5 to 9 days for placebo | 7.92 days of ICU stay (95% CrI 6.79 to 9.37) corresponding to 8 days in the placebo group | RoM: exp(0.01) = 1.01 (95% CI 0.90 to 1.14); log RoM: 0.01 (‐0.11 to 0.13); I² = 0% | 618 (4 studies) | ⊕⊕⊕⊝ Moderatea | RoM (95% CrI): 0.99 (0.85 to 1.17), SUCRA = 0.496, mean Pr(best) = 0.014, mean rank = 4.02 |
| Atypical antipsychotic vs placebo | Median length of ICU stay: 3 to 16 days for placebo | 7.40 days of ICU stay (95% CrI 6.37 to 8.66) corresponding to 8 days in the placebo group | RoM: exp(‐0.09) = 0.91 (95% CI 0.84 to 1.00); log RoM: ‐0.09 (‐0.18 to ‐0.00); I² = 0% | 577 (4 studies) | ⊕⊕⊕⊕ High | RoM (95% CrI): 0.92 (0.80 to 1.08), SUCRA = 0.709, mean Pr(best) = 0.106, mean rank = 2.75 |
| Statin (HMG‐CoA) vs placebo | Mean length of ICU stay: 13 days for placebo | 8.54 days of ICU stay (95% CrI 6.46 to 11.25) corresponding to 8 days in the placebo group | RoM: exp(0.06) = 1.06 (95% CI 0.91 to 1.23); log RoM: 0.06 (‐0.09 to 0.21); I² not applicable | 272 (1 study) | ⊕⊕⊝⊝ Lowa,b | RoM (95% CrI): 1.07 (0.81 to 1.41), SUCRA = 0.344, mean Pr(best) = 0.030, mean rank = 4.93 |
| Alpha2 agonist vs placebo | Median length of ICU stay: 7.5 days for placebo | 6.43 days of ICU stay (95% CrI 4.42 to 9.33) corresponding to 8 days in the placebo group | RoM: exp(‐0.22) = 0.80 (95% CI 0.59 to 1.08); log RoM: ‐0.22 (‐0.53 to 0.08); I² not applicable | 71 (1 study) | ⊕⊕⊝⊝ Lowa,b | RoM (95% CrI): 0.80 (0.55 to 1.17), SUCRA = 0.853, mean Pr(best) = 0.608, mean rank = 1.88 |
| Cholinesterase inhibitor vs placebo | Median length of ICU stay: 8 days for placebo | 17.53 days of ICU stay (95% CrI 11.76 to 26.14) corresponding to 8 days in the placebo group | RoM: exp(0.78) = 2.18 (95% CI 1.58 to 3.03); log RoM: 0.78 (0.46 to 1.11); I² not applicable | 104 (1 study) | ⊕⊕⊕⊝ Moderatea | RoM (95% CrI): 2.19 (1.47 to 3.27), SUCRA = 0.002, mean Pr(best) = 0, mean rank = 6.99 |
| Opioid vs placebo | No study reported this comparison | 7.40 days of ICU stay (95% CrI 4.95 to 11.24) corresponding to 8 days in the opioid group | Pairwise meta‐analysis not performed | 0 (0 studies) |
⊕⊕⊝⊝ Very lowa,c | RoM (95% CrI): 0.92 (0.62 to 1.40), SUCRA = 0.639, mean Pr(best) = 0.238, mean rank = 3.17 |
| *The basis for the assumed risk (e.g. the median control group risk across studies). The corresponding risk (and its 95% CrI) is calculated as the assumed risk multiples the ratio of means (and its 95% CrI) based on NMA. CI: confidence interval; CrI: credible interval; HMG‐CoA: 5‐hydroxy‐3‐methylglutaryl‐coenzyme A reductase inhibitor; NMA: network meta‐analysis; Pr(best): probability(best); RoM: ratio of means; SUCRA: surface under the cumulative ranking curve. | ||||||
| GRADE Working Group grades of evidence. High quality: further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: we are very uncertain about the estimate. | ||||||
aDowngraded one level for imprecision (wide credible interval). bDowngraded one level for single trial with risk of bias and indirectness.
cDowngraded two levels for only indirect evidence available and risk of bias of a single trial informing opioid vs typical antipsychotic.
Summary of findings 5. Length of hospital stay.
| Outcome: length of hospital stay | ||||||
|
Patient or population: critically ill adult with confirmed or at high risk of delirium
Settings: intensive care units in Australia and New Zealand, Canada, Egypt, Netherlands, Turkey, USA, UK
Intervention: any pharmacological intervention Control: placebo or active comparator | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) |
Ratio of means (RoM) based on log RoM estimates from meta‐analysis (IV, random, 95% CI) |
Number of participants (studies) | Quality of the evidence (GRADE) based on NMA | NMA results (assuming consistency equations) | |
| Assumed risk | Corresponding risk based on NMA estimates | |||||
| Placebo/Comparator | Intervention drug | |||||
| Typical AP vs placebo | Median length of hospital stay: 13 to 26 days for placebo | 16.48 days of hospital stay (95% CrI 11.74 to 21.29) corresponding to 18 days in the placebo group | RoM: exp(‐0.12) = 0.89 (95% CI 0.68 to 1.15); log RoM: ‐0.12 (‐0.38 to 0.14); I² = 72% | 479 (2 studies) | ⊕⊕⊝⊝ Lowa,b | RoM (95% CrI): 0.92 (0.65 to 1.18), SUCRA = 0.722, mean Pr(best) = 0.235, mean rank = 2.67 |
| Atypical AP vs placebo | Median length of hospital stay: 6 to 26 days for placebo | 16.69 days of hospital stay (95% CrI 12.47 to 20.79) corresponding to 18 days in the placebo group | RoM: exp(‐0.04) = 0.96 (95% CI 0.88 to 1.05); log RoM: ‐0.04 (‐0.13 to 0.05); I² = 0% | 511 (3 studies) | ⊕⊕⊕⊝ Moderateb |
RoM (95% CrI): 0.93 (0.69 to 1.16), SUCRA = 0.693, mean Pr(best) = 0.218, mean rank = 2.84 |
| Statin (HMG‐CoA) vs placebo | Mean length of hospital stay: 22 to 23.1 days for placebo | 17.55 days of hospital stay (95% CrI 12.45 to 23.47) corresponding to 18 days in the placebo group | RoM: exp(‐0.01) = 0.99 (95% CI 0.88 to 1.13); log RoM: ‐0.01 (‐0.13 to 0.12); I² = 0% | 369 (2 studies) |
⊕⊕⊕⊝ Moderateb |
RoM (95% CrI): 0.98 (0.69 to 1.30), SUCRA = 0.537, mean Pr(best) = 0.147, mean rank = 3.78 |
| Alpha2 agonist vs placebo | Median length of hospital stay: 12.5 days for placebo | 19.80 days of hospital stay (95% CrI 12.37 to 31.52) corresponding to 18 days in the placebo group | RoM: exp(0.09) = 1.09 (95% CI 0.84 to 1.42); log RoM: 0.09 (‐0.17 to 0.35); I² not applicable | 71 (1 study) |
⊕⊕⊕⊝ Moderatec |
RoM (95% CrI): 1.10 (0.69 to 1.75), SUCRA = 0.301, mean Pr(best) = 0.090, mean rank = 5.19 |
| Cholinesterase Inhibitor vs placebo | Median length of hospital stay: 25 days for placebo | 20.00 days of hospital stay (95% CrI 12.64 to 31.93) corresponding to 18 days in the placebo group | RoM: exp(0.11) = 1.12 (95% CI 0.86 to 1.43); log RoM: 0.11 (‐0.15 to 0.36); I² not applicable | 104 (1 study) |
⊕⊕⊕⊝ Moderatec |
RoM (95% CrI): 1.11 (0.70 to 1.77), SUCRA = 0.280, mean Pr(best) = 0.078, mean rank = 5.32 |
| Opioid vs placebo | No study reported this comparison | 17.51 days of hospital stay (95% CrI 9.89 to 28.78) corresponding to 18 days in the opioid group | Pairwise meta‐analysis not performed | 0 (0 studies) |
⊕⊕⊝⊝ Very lowb,d | RoM (95% CrI): 0.97 (0.55 to 1.60), SUCRA = 0.532, mean Pr(best) = 0.225, mean rank = 3.81 |
| *The basis for the assumed risk (e.g. the median control group risk across studies). The corresponding risk (and its 95% CrI) is calculated as the assumed risk multiples the ratio of means (and its 95% CrI) based on NMA. CI: confidence interval; CrI: credible interval; HMG‐CoA: 5‐hydroxy‐3‐methylglutaryl‐coenzyme A reductase inhibitor; NMA: network meta‐analysis; Pr(best): probability(best); RoM: ratio of means; SUCRA: surface under the cumulative ranking curve. | ||||||
| GRADE Working Group grades of evidence. High quality: further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: we are very uncertain about the estimate. | ||||||
aDowngraded one level for heterogeneity (I² of 50% to 75%, > 75% considered as medium and large heterogeneity). bDowngraded one level for imprecision (wide credible interval). cDowngraded one level for single small trial with risk of bias and indirectness. dDowngraded two levels for only indirect evidence available and risk of bias of a single trial informing opioid vs typical antipsychotic.
Summary of findings 6. QTc prolongation.
| Outcome: QTc prolongation | ||||||
|
Patient or population: critically ill adult with confirmed or at high risk of delirium
Settings: intensive care units in Australia and New Zealand, Canada, Egypt, Netherlands, Turkey, USA, UK
Intervention: any pharmacological intervention Control: placebo or active comparator | ||||||
| Comparisons | Illustrative comparative risks* (95% CI) |
Relative effect OR (95% CI) |
Absolute effect (auto calculation using GRADEpro GDT) |
Number of participants (studies) | Quality of the evidence (GRADE) | |
| Assumed risk | Corresponding risk | |||||
| Placebo/Comparator | Intervention drug | |||||
| Typical antipsychotic vs placebo | 62 per 1000 | 78 per 1000 | 1.26 (0.68 to 2.34) I² = 0% | 15 more per 1000 (from 19 fewer to 72 more) | 656 (4 studies) | ⊕⊕⊕⊕ High |
| Atypical antipsychotic vs placebo | 90 per 1000 | 118 per 1000 | 1.28 (0.45 to 3.66) I² = 56% | 22 more per 1000 (from 48 fewer to 176 more) | 577 (4 studies) | ⊕⊕⊕⊝ Moderatea |
| Typical antipsychotic vs atypical antipsychotic | 114 per 1000 | 66 per 1000 | 0.55 (0.28 to 1.08) I² = 0% | 48 fewer per 1000 (from 79 fewer to 8 more) | 447 (2 studies) | ⊕⊕⊕⊕ High |
| Alpha2 agonist vs typical antipsychotic | 400 per 1000 | 400 per 1000 | 1.00 (0.17 to 5.98) I² not applicable | 0 fewer per 1000 (from 298 fewer to 399 more) | 20 (1 study) | ⊕⊕⊝⊝ Lowb |
| Alpha2 agonist vs 5HT3 inhibitor | 0 per 1000 | 0 per 1000 | OR not estimable I² not applicable | Not estimable | 64 (1 study) | ⊕⊕⊝⊝ Lowb |
| *The basis for the assumed risk (e.g. the median control group risk across studies). The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; OR: odds ratio. | ||||||
| GRADE Working Group grades of evidence. High quality: further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: we are very uncertain about the estimate. | ||||||
aDowngraded one level for heterogeneity (I² of 50% to 75%, > 75% considered as medium and large heterogeneity).
bDowngraded two levels for imprecision (wide confidence interval, single small trial with risk of bias).
Background
Description of the condition
Delirium is a reversible, non‐specific syndrome of cognitive impairment commonly associated with surgery, infection, or critical illness (APA 2013). In the intensive care unit (ICU), this acute brain dysfunction is reported in 40% to 60% of non‐ventilated patients, and in 50% to 80% of mechanically ventilated patients (Ely 2001a; Ely 2001b; Ely 2007; Hipp 2012; Inouye 2014). Delirium is challenging to detect, as symptoms are highly variable, with either hyperactivity or hypoactivity, or even a mixed picture, and symptoms fluctuate with periods of lucidity (Inouye 2014). Delirium may be detected by psychiatric assessment based on the Diagnostic and Statistical Manual (DSM) criteria (APA 2013), or by use of a validated screening tool (Bergeron 2001; Ely 2001a; Neelon 1996); however, assessment in the ICU is predicated on the patient being awake and able to communicate, and delirium is said to be "unable to be assessed" when the patient does not respond to verbal communication. In the ICU, commonly used sedatives and opioids impair consciousness, thereby making identification of delirium challenging (Patel 2014). Drug exposure should be considered when ICU delirium is assessed, and if possible, assessments should be co‐ordinated with periods of wakefulness or should be conducted during a sedation interruption (Patel 2014).
Over the past decade, we have acquired a greater understanding of the effects of delirium on patients, their families, and the healthcare system. Clinically important outcomes of delirious critically ill patients include prolonged duration of mechanical ventilation and ICU and hospital stay, as well as long‐term cognitive impairment, increased likelihood of transfer to long‐term care facilities, and mortality (Black 2011; Ely 2001b; Ely 2004; Girard 2010b; Jackson 2004; Lin 2004; Milbrant 2004; Pisani 2009; Van den Boogaard 2012). The odds of a poor outcome with delirium are increased by patient frailty, advanced age (> 75 years), pre‐existing cognitive impairment, and visual or hearing impairment (Andrew 2006; Inouye 2006a). Precipitating factors are numerous and include sleep deprivation, pain, environmental insults (e.g. noise, physical restraint use, catheters), and psychoactive drug exposure (e.g. sedatives) (Burry 2017; Fraser 2013; Inouye 2006a; Rose 2016; Zaal 2015).
Description of the intervention
Pharmacological interventions for delirium treatment have focused on alterations in neurotransmitter pathways, in particular dopaminergic and cholinergic pathways. At present, the pathophysiology of delirium is not fully understood (Gunther 2008; Reade 2014). Hypotheses currently include abnormalities in cerebral oxidative metabolism, direct neurotoxic effects of inflammatory cytokines, such as those released during sepsis and septic shock, and alterations in neurotransmitters that modulate cognition, behaviour, and mood (e.g. cholinergic, dopaminergic, serotonergic, gamma‐aminobutyric acid (GABA) pathways) (Cerejeira 2011; de Rooji 2007; Ebersoldt 2007; Flacker 1999; Gunther 2008; Inouye 2006b; Rudolph 2008; White 2002). These pathophysiological mechanisms are not thought to be mutually exclusive and are likely to act together.
In the light of these different proposed mechanisms, it is not surprising that numerous pharmacological strategies for delirium have been investigated, including alpha2 agonists, antidepressants, antipsychotic drugs (either typical or atypical agents), benzodiazepines, cholinesterase inhibitors, melatonin and melatonin agonists, and opioids (Devlin 2010; Girard 2010a; Maldonado 2009; Ohta 2013; Reade 2009; Rubino 2010; van Eijk 2010). In considering these agents, it is important to note that critical care guidelines first recommend the use of non‐pharmacological strategies in both prevention and management of delirium (Barr 2013). These non‐pharmacological strategies include early mobilization and re‐orientation, risk factor assessment and modification (e.g. drugs, medical devices), and normalization of the sleep‐wake cycle (e.g. noise reduction, use of ear plugs) (Inouye 2006a; Schweickert 2009). Guidelines suggest that when delirium is suspected or identified, patients should be closely evaluated for identification of underlying cause(s), allowing for exposure to be removed or corrected whenever possible; pharmacological interventions are to be used only when non‐pharmacological methods have failed to control symptoms (Barr 2013).
How the intervention might work
Given the multiple neurotransmitters linked to development of delirium, pharmacological strategies have investigated target suspected neurotransmitter imbalances or attempts to control distressing cognitive (e.g. hallucinations) or dangerous behaviours (e.g. agitation, interference with medical devices). Pharmacological strategies may target pain control (e.g. opioids) or the dopaminergic (e.g. antipsychotics), cholinergic (e.g. cholinesterase inhibitors), GABA (e.g. benzodiazepines), N‐methyl‐D‐aspartate (NMDA) (e.g. ketamine), serotonergic (e.g. antidepressants, antinauseants, melatonin), and alpha2 (e.g. clonidine, dexmedetomidine) pathways (Devlin 2010; Girard 2010a; Maldonado 2009; Ohta 2013; Reade 2009; Rubino 2010; van Eijk 2010). The specific therapeutic effects of such agents are unknown, but effects may be mediated through their ability to affect sedation and behavioural symptoms.
Despite conflicting evidence for the benefits of various pharmacological interventions, many of these agents are routinely used to treat ICU delirium, or to at least manage symptoms (e.g. agitation), and they are often continued after hospital discharge (Bell 2007; MacSweeney 2009). Of the available pharmacological strategies, antipsychotics represent the most common treatment for ICU delirium, despite limited evidence regarding their benefit and studies in non‐critically ill patients identifying significant adverse effects, including sudden death (Barr 2013; Briskman 2010; Burry 2014; Gill 2007; MacSweeney 2009; Tropea 2009; Wang 2005).
Why it is important to do this review
ICU delirium is associated with prolonged duration of mechanical ventilation and ICU and hospital stay, as well as increased mortality (Ely 2001b; Ely 2004; Girard 2010b; Jackson 2004; Lin 2004; Milbrant 2004; Pisani 2009; Van den Boogaard 2012). ICU delirium initiates a cascade of events that can include functional decline and long‐term cognitive impairment, with resultant caregiver burden (Girard 2010b; Jackson 2004; Van den Boogaard 2012). The geriatric and oncological literature shows that delirium is traumatic for both patients and family members, and it can lead to long‐term psychological sequelae (Bruera 2009; Morita 2004; Partridge 2013; Rosenbloom‐Brunton 2010). The economic burden of delirium is also significant; each additional day spent in a delirious state is associated with a 20% increased risk of prolonged hospitalization, translating to an average of more than 10 additional hospital days per patient. The annual cost of delirium is estimated to be greater than USD 164 billion in the USA, and greater than EUR 182 billion as estimated across 18 European countries (Leslie 2008; OECD 2012; WHO Regional Office 2012). Furthermore, delirium is considered a substantial public health concern that has garnered the attention of patient safety institutes; it is now included as an indicator of quality care for the elderly (IHI 2015).
Advances in detection of ICU delirium and improved understanding of its impact on patient outcomes have prompted trials comparing different treatment options (both pharmacological and non‐pharmacological), either against each other or versus placebo. However, there remains considerable uncertainty regarding the relative benefits and safety of pharmacological interventions for the ICU population, and trials have shown benefit (Devlin 2010; Pandharipande 2007; Reade 2009), indeterminate outcomes (Girard 2010a; Page 2013), or harm (van Eijk 2010). A previous Cochrane Review on antipsychotics for delirium did not specifically address the ICU population (Lonergan 2007); numerous ICU‐specific trials have been published since this review was completed. A recent systematic review of ICU delirium included both prevention and treatment studies (Al‐Qadheeb 2014), as well as randomized controlled trials (RCTs) evaluating sedation strategies, in which delirium was evaluated as a secondary endpoint when the study population considered was not restricted to patients with confirmed delirium. As a Cochrane Review protocol by Herling and colleagues will provide data on delirium prevention trials in critically ill adult patients (Herling 2018), our review focuses on delirium treatment trials in critically ill adult patients.
Given the availability of numerous strategies to treat ICU delirium in clinical practice, and the existence of many trials yielding conflicting results, we planned this systematic review to include a network meta‐analysis (NMA) to determine the comparative benefits and harms of all published pharmacological interventions for treatment of delirium based on available direct and indirect evidence of relevance. An NMA, also known as a multiple treatment comparison meta‐analysis, is a statistical method used to assess the comparative effectiveness of multiple different interventions among similar patient populations that have not been compared directly in an RCT. In contrast to conventional pairwise meta‐analysis (e.g. RCTs comparing treatment A vs treatment B), NMAs can provide estimates of relative efficacy between all interventions, even though some have never been compared head‐to‐head via indirect evidence (i.e. comparing results from two or more studies that have one treatment in common).
Objectives
Primary objective
To assess the effects of pharmacological interventions for treatment of delirium on duration of delirium in critically ill adults with confirmed or documented high risk of delirium
Secondary objectives
To assess the following:
effects of pharmacological interventions on delirium‐free and coma‐free days; days with coma; delirium relapse; duration of mechanical ventilation; ICU and hospital length of stay; mortality; and long‐term outcomes (e.g. cognitive; discharge disposition; health‐related quality of life); and
the safety of such treatments for critically ill adult patients.
Methods
Criteria for considering studies for this review
Types of studies
We sought randomized controlled trials (RCTs), including quasi‐RCTs (i.e. when the method of allocation was not strictly random, such as by alternation, date of birth, or case record number), and RCTs with an open‐label study design. We excluded non‐RCT study designs due to their potential for bias and the anticipated availability of RCTs.
Types of participants
We sought RCTs designed to examine pharmacological interventions for treatment of delirium in critically ill adults. We defined critically ill patients as those treated in an ICU of any specialty (e.g. burn, cardiac, medical, surgical, trauma) or high‐dependency unit. We included trials in which a trained individual (e.g. psychiatrist) evaluated participants for delirium using the Diagnostic and Statistical Manual of Mental Disorders (DSM) criteria (APA 2013), or using a validated delirium assessment tool (e.g. Confusion Assessment Method for the ICU (CAM‐ICU), Intensive Care Delirium Screening Checklist (ICDSC), Neelon and Champagne (NEECHAM) Confusion Scale, Delirium Rating Scale, or Delirium Rating Scale‐revised‐98) (Bergeron 2001; Ely 2001b; Neelon 1996; Trzepacz 2001). We also included RCTs that treated subsyndromal delirium (i.e. some features of delirium), as these patients are considered to be at high risk of transitioning to delirium and are often included in ICU delirium treatment studies.
Types of interventions
We sought delirium treatment RCTs that compared use of any pharmacological (drug) to treat delirium including alpha2 agonists (e.g. clonidine, dexmedetomidine), antidepressants (e.g. fluoxetine), antipsychotics (either typical (e.g. haloperidol) or atypical agents (e.g. quetiapine)), benzodiazepines (e.g. lorazepam), cholinesterase (CHE) inhibitors (e.g. rivastigmine), N‐methyl‐D‐aspartate (NMDA) receptor antagonist (e.g. ketamine), melatonin and melatonin agonists (e.g. ramelteon), opioids (e.g. morphine), propofol, serotonin receptor antagonists (e.g. ondansetron), and statins (e.g. atorvastatin) versus another active drug treatment, a placebo, or a non‐pharmacological intervention (e.g. mobilization). We did not apply any restrictions in terms of drug class, dose, route of administration, or duration of delirium or drug exposure.
Our hypothetical network structure published in the protocol was designed to be analysed at the drug class level and illustrated a network diagram of 'nodes' (i.e. drug classes) and 'edges' (i.e. comparisons between different drug classes from existing trials) (Burry 2015), thus describing a treatment network of all possible comparisons between drug classes. The extent to which trial data are available along the 'edges' for each outcome will depend upon the search results.
Types of outcome measures
Primary outcomes
Duration of delirium (defined as the time from which delirium was identified or the patient was randomized until resolution (i.e. screened negative as defined by study authors)), measured in days
Secondary outcomes
Delirium‐free and coma‐free days (to 14, 21, 28 days) and days with coma (reported in days)
Relapse of delirium (reported as a proportion)
Resolution of delirium symptoms (e.g. hallucinations, agitation)
Duration of mechanical ventilation (days)
Length of stay (ICU and hospital) (days)
Mortality (e.g. 30‐day, 60‐day, 90‐day, ICU, hospital, following hospital discharge, and one year as reported by study authors)
Use of physical restraint
Hospital discharge disposition (e.g. chronic care facility, home)
Long‐term cognitive outcomes (e.g. change in Mini Mental Status Exam) as reported by study authors
Health‐related quality of life (as reported by study authors)
Adverse drug events (e.g. akathisia, arrhythmias, extrapyramidal side effects, seizures)
Search methods for identification of studies
We sought to identify all eligible trials regardless of publication status through systematic and sensitive search strategies as outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We did not impose any language or publication restrictions.
Electronic searches
Our electronic search strategies were developed and tested through an iterative process with an experienced medical information specialist (Appendix 1; Appendix 2; Appendix 3; Appendix 4). The search strategies utilized a combination of controlled vocabulary terms (e.g. ICU, delirium) and keywords (e.g. ICU, acute brain dysfunction). We used a validated RCT filter and a filter that limited studies to humans. We searched the following electronic databases from their inception date to 21 March 2019: Ovid MEDLINE ALL®, Embase Classic+Embase, and PsycINFO using OVID platform. We also searched the Cochrane Library on Wiley, the International Prospective Register of Systematic Reviews (PROSPERO; http://www.crd.york.ac.uk/PROSPERO/), the Cumulative Index to Nursing and Allied Health Literature (CINAHL), and Web of Science. We adjusted search vocabulary and syntax for each database. The core strategy was reviewed prior to execution by another senior information specialist using the Peer Review for Electronic Search Strategies (PRESS) template (Sampson 2009).
We performed a separate search for published systematic reviews to identify additional published or unpublished trials. We performed a grey literature search of relevant databases and websites using resources listed in Grey Matters (http://www.cadth.ca/en/resources/finding‐evidence‐is/grey‐matters) developed by the Canadian Agency for Drugs and Technologies in Health (CADTH). Last, we scanned the reference lists of all included studies and any relevant reviews on delirium treatment to identify additional studies.
Searching other resources
We hand searched the citations of all included studies and any systematic reviews identified. We searched abstracts from annual scientific meetings of the Society of Critical Care Medicine, the European Society of Intensive Care Medicine, the International Symposium on Intensive Care and Emergency Medicine, the American Delirium Society, the American Thoracic Society, Chest, and the Australian and New Zealand Intensive Care Society from 2011 to 2019 to identify studies not yet published in full. We also searched for unpublished and ongoing trials on the following websites using the term "delirium".
Data collection and analysis
Selection of studies
Two review authors (LB, LR) independently screened all retrieved titles and abstracts using the selection criteria described in the protocol (Burry 2015). Next, these two review authors (LB, LR) independently reviewed selected full‐text articles to determine inclusion. We resolved disagreements by discussion, without the need to refer to the assigned independent arbiter (EWE). References were managed in the software package EndNote (Endnote Version X6, Thomson Reuters, Carlsbad, CA, USA), and we documented the reasons for exclusion in the notes field. We documented the process of study selection using a PRISMA flow diagram (Moher 2009).
Data extraction and management
We extracted data from the included trials using a standardized electronic form (Microsoft Corporation, Redmond, WA, USA). Four review authors (DW, SM, NA, IE) worked independently to extract data; two review authors were assigned to each study. Data extractors were not blinded to the identity of study authors. We extracted data related to publication (e.g. journal reference, study authors, year of publication), study design (e.g. number of centres, country, methods of enrolment, randomization, allocation concealment, blinding), patient demographics (e.g. age, sex, severity of illness score, reasons for admission), interventions (e.g. drug, mode of administration, dose, how titrated, who administered, use of rescue medications for agitation), delirium and sedation assessment (e.g. method, who assessed), co‐interventions that might alter duration delirium, stay or mechanical ventilation (e.g. ventilator weaning strategies, type of sedative or analgesic, early mobilization), and our selected outcomes. We also extracted data on management of missing data, reporting of outcomes, type of analysis performed (e.g. intention to treat), and other potential sources of bias (e.g. funding source, referral bias). When necessary, we (LB) contacted the study corresponding author to clarify issues related to data reporting or to obtain further study details. Data extraction was confirmed and discrepancies between review author pairs resolved by an arbiter (LB). Checked data were then entered into Review Manager 5 by one review author (WC) and were double‐checked by two review authors (BH, LB) (Review Manager 2014).
Assessment of risk of bias in included studies
Each data extractor (DW, SM, NA, IE) independently assessed risk of bias for his/her assigned studies. A third review author (LB) verified each assessment. Risk of bias was determined via a domain‐based evaluation that was included in the data extraction form, and as recommended by Cochrane (Higgins 2011). The domains were as follows.
Random sequence generation (i.e. selection bias).
Allocation concealment (i.e. selection bias).
Blinding of participants and personnel (i.e. performance bias).
Blinding of outcomes assessment (i.e. detection bias).
Incomplete outcome data (i.e. attrition bias).
Selective reporting.
Other bias (e.g. study source of funding, role of the sponsor, referral bias).
For each domain, we explicitly judged the risk of bias as high, low, or unclear. We assigned domains 'unclear' if detail was insufficient to determine risk, or if risk of bias was unclear or unknown. We judged incomplete outcome data as low risk of bias when causes of dropout were similar and numbers were balanced between study groups and less than 15%. We generated a risk of bias graph and summary upon completion of assessment.
Measures of treatment effect
For all continuous outcomes (duration of delirium, duration of ventilation, hospital length of stay, ICU length of stay, delirium‐free and coma‐free days, coma days), more than half of the included studies reported medians and interquartile ranges (IQRs) as opposed to means and standard deviations (SDs), standard errors (SEs) or confidence intervals (CIs). We converted medians and IQRs to means and SDs according to methods described elsewhere (Wan 2014). Due to the skewed nature of these outcomes, we transformed means and SDs to the log scale using methods outlined previously (Higgins 2008). For continuous outcomes, the mean difference (MD) between two interventions on the log scale equals the log ratio of means (log RoM); after exponentiation, estimates can be interpreted as the RoM of two interventions. Evidence synthesis on the log RoM scale allows continuous outcomes measured within various lengths of time windows across studies. Findings for binary outcomes were expressed in terms of odds ratios (ORs).
Based on mean and SD values following transformation, fixed‐effect and random‐effects NMA models with Normal Likelihood and the identify link were fit to the data (Dias 2011b). We present comparisons between interventions in terms of RoM (RoM: mean[expt]/mean[ctrl]) with 95% credible intervals (CrI). Values of RoM < 1 favour the active intervention, whereas values of RoM > 1 favour the placebo or comparator for all continuous outcomes except for delirium‐free and coma‐free days. For dichotomous outcome measures, both fixed‐effect and random‐effects NMA models with binomial likelihood were fit to the data, with comparisons between interventions expressed in terms of ORs with 95% CrI.
For each outcome, NMA enabled us to calculate the probability for each intervention to be at each possible rank. The Surface Under the Cumulative RAnking curve (SUCRA) value, the mean rankings (with 2.5% and 97.5% quantiles) of each intervention, and the probability of each intervention to be the best (referred to hereafter as 'Pr(best)') were also estimated (Salanti 2011). Pr(best) and SUCRA values range between 0 and 1, with values nearer 1 indicative of preferred treatments. Values of smaller mean rank also suggest preferred treatments. Further details regarding the methods and implementation of NMA are provided in the published protocol (Burry 2015).
Unit of analysis issues
We used individual study participants in each trial arm as the unit of analysis. We included all interventions relevant to this review. If a trial involves multiple arms of the same drug class (e.g. multiple atypical antipsychotics) compared to a control group, we planned to merge data from the same drug class for pairwise comparisons. Neither cluster‐randomized trials nor cross‐over trials were identified through the literature search. We did not anticipate cross‐over trials to evaluate delirium in the ICU, as this study design is not typically used in the ICU.
Dealing with missing data
We conducted meta‐analyses based on data available from our included studies. For missing SDs associated with continuous outcomes, we first contacted study authors for more information; we made a maximum of three attempts.
Assessment of heterogeneity
An important aspect of NMA is examining included studies to determine if they are sufficiently similar in terms of study design and patient population. We describe each included trial in the Characteristics of included studies tables. Within a treatment network involving multiple interventions, heterogeneity can be the result of an uneven distribution of important clinical and methodological effect modifiers across studies or across comparisons. We assessed the presence of statistical heterogeneity by visual inspection of forest plots and by calculation of the I² statistic (Higgins 2003), as well as by the Chi² test for homogeneity (P < 0.10 deemed significant). If the I² statistic was > 50%, we assessed the types and sources of heterogeneity (clinical and methodological). We qualitatively assessed clinical heterogeneity by examining additional delirium management strategies used in each trial (e.g. use of rescue medications or physical restraints to manage severe agitation, non‐drug strategies such as noise reduction or early mobilization). We also assessed clinical heterogeneity by examining factors that may influence delirium and sedation practices (for example, types of sedatives and analgesics used, use of drugs known to increase the risk of delirium, e.g. benzodiazepines, and definitions of outcomes assessed).
Assessment of reporting biases
Reporting biases can occur due to an increased likelihood of positive (demonstration of effect) trials (large or small) being published compared to negative (no effect demonstrated) trials. It is difficult to estimate the number of unpublished delirium trials. For direct comparisons in the network where a minimum of 10 studies were available, we reviewed comparison‐adjusted funnel plots to assess for small‐study effects as signals of publication bias (Salanti 2014).
Data synthesis
Methods for direct treatment comparisons
We performed conventional pairwise meta‐analyses in Review Manager 5.3 for all outcomes and comparisons that had at least two studies available (Review Manager 2014). A variation of the inverse‐variance random‐effects model was applied to continuous outcomes (DerSimonian 1986), whereas the Mantel‐Haenszel random‐effects model was applied to binary outcomes (DeMets 1987), allowing for variation within and between studies.
Methods for network meta‐analysis (mixed treatment comparisons)
NMA is a method of synthesizing evidence from trials addressing the same question but involving multiple different interventions. NMA combines direct and indirect evidence across a network of RCTs into a single effect size for each pair of interventions. For a given comparison (e.g. A vs B), direct evidence was provided by studies that compared two treatments head‐to‐head. Indirect evidence for this comparison was provided by studies that compared A versus C and B versus C (Caldwell 2005; Higgins 1996).
We followed established procedures to assess the validity of the assumptions of homogeneity, similarity, and consistency (Donegan 2013). We performed NMAs within a Bayesian framework, assuming a common between‐study variance parameter across all comparisons and accounting for correlations in multi‐arm studies (Lu 2006; Salanti 2011). A vague prior distribution for the between‐study variance parameter (specifically, Uniform (0, 3)) and vague prior distribution for log ratio of means between each intervention compared with placebo (specifically, Normal (0, 100)) were used for all analyses. We reported findings when using the most recent PRISMA Extension Statement for NMA (Hutton 2015). Two review authors (WC, BH) performed NMAs with OpenBUGS software (version 3.2.3, MRC Biostatistics Unit, Cambridge, UK) (Lunn 2000; Spiegelhalter 2014). We expressed findings for continuous outcomes in terms of RoMs and findings for binary outcomes in terms of ORs with corresponding 95% CrI (Dias 2011a; Dias 2011b; Dias 2013). Network diagrams were drawn to depict the evidence for each outcome. In the network diagrams, the size of the treatment nodes reflects the number of participants randomized to each treatment, and the thickness of the edges reflects the number of studies informing each comparison.
We evaluated the adequacy of model fit by comparing the total residual deviance to the number of unconstrained data points (i.e. the total number of study arms); fit was adequate if these quantities were close. Based on mean and SD values following transformation, fixed‐effect and random‐effects NMA models with Normal Likelihood and the identity link were fit to the data (Dias 2011b). Both fixed‐effect (FE) and random‐effects (RE) consistency models were fit, and we compared these models using the Deviance Information Criterion (DIC), with lower value indicating better model fit (Spiegelhalter 2002). We considered a difference of five points or more indicative of an important difference. We also fit unrelated means models to the data and compared DIC values and posterior mean deviance contributions with those from consistency models to detect violations of the consistency assumption. We assessed model convergence with established methods including inspection of Gelman‐Rubin‐Brooks diagnostics and potential scale reduction factors (Brooks 1998; Gelman 1996). As described earlier, we also estimated SUCRA values, mean rankings, and Pr(best) values for each intervention (Salanti 2011). For additional analyses, we planned to explore the impact of certain study characteristics through subgroup analyses or meta‐regression.
Subgroup analysis and investigation of heterogeneity
We planned to explore subgroup analyses or meta‐regression analyses or both, to assess the impact of covariates on findings to establish their robustness, if sufficient studies were available; specifically:
age (< 65 years, ≥ 65 years);
different ICU populations (e.g. medical only, surgical only);
delirium subtype (e.g. hyperactive, hypoactive, mixed); or
use of co‐interventions with non‐drug approaches (e.g. noise reduction, music therapy, early mobilization).
Sensitivity analysis
We planned to consider sensitivity analyses involving alternative geometries of the network. Planned re‐formulations of the network included:
excluding studies with high risk of bias;
collapsing atypical and typical antipsychotics into one node;
splitting each node to reflect ‘low dose’ and ‘high dose’, based on the median dose reported in trials; and
splitting each node to reflect fixed dosing and PRN (pro re nata or as needed) only dosing.
We explored additional analyses after excluding studies that focused on subsyndromal delirium.
'Summary of findings' tables and GRADE
In the 'Summary of findings' tables, we present the specific review outcomes duration of delirium, delirium‐free and coma‐free days, days with coma, duration of mechanical ventilation, length of ICU, and length of hospital stay, as recommended by Cochrane (Higgins 2011; Schunemann 2011; Yepes‐Nunez 2019). We used the GRADE approach (https://gradepro.org/) to assess the quality of the evidence for comparisons based on NMA. We graded the quality of evidence for each outcome as 'high', 'moderate', 'low', or 'very low' using GRADEPro software (GRADEpro GDT), after considering trial limitations (randomization, allocation concealment, and blinded outcome assessment), within‐study directness of evidence, heterogeneity, precision of effect estimates, and indirectness. We did not assess risk of publication bias/small‐study effects through funnel plots given the small number of studies available for any pairwise comparison. When we identified an issue that we considered to be serious for each of the GRADE criteria, we downgraded the quality of evidence and justified our decision in the table footnotes. We assessed the extent of heterogeneity (i.e. I² statistic) and examined imprecision based on the width of the CI for treatment effect estimates.
Results
Description of studies
Results of the search
See Characteristics of included studies,Characteristics of excluded studies,Characteristics of studies awaiting classification, and Characteristics of ongoing studies.
The results of our search are outlined in Figure 1. The electronic database search yielded 7658 citations, and we identified an additional 16 records through other sources. After we removed duplicate items, 4461 unique citations remained. We excluded 4076 studies based on title and abstract, and we assessed the remaining 385 papers as full text. Fourteen studies met our inclusion criteria (Al‐Qadheeb 2016; Atalan 2013; Bakri 2015; Devlin 2010; Girard 2010a; Girard 2018; Hakim 2012; Needham 2016; Page 2013; Page 2017; Reade 2009; Reade 2016; Skrobik 2004; van Eijk 2010). Six studies await classification (NCT02366299; NCT00429676; Emerson 2014; Peters 2015; Schoeffler 2012; ISRCTN33122761) ‐ three as conference abstracts (Emerson 2014; Peters 2015; Schoeffler 2012), and three as trial registrations (NCT02366299; NCT00429676; ISRCTN33122761). Ten studies are ongoing (NCT01811459; NCT03317067; NCT02807467; NCT02216266; NCT02343575; NCT00351299; NCT03628391; IRCT20121231011956N10; IRCT20180911040998N1; NCT03392376), two of which have published protocols (Louis 2018; Hollinger 2017).
1.
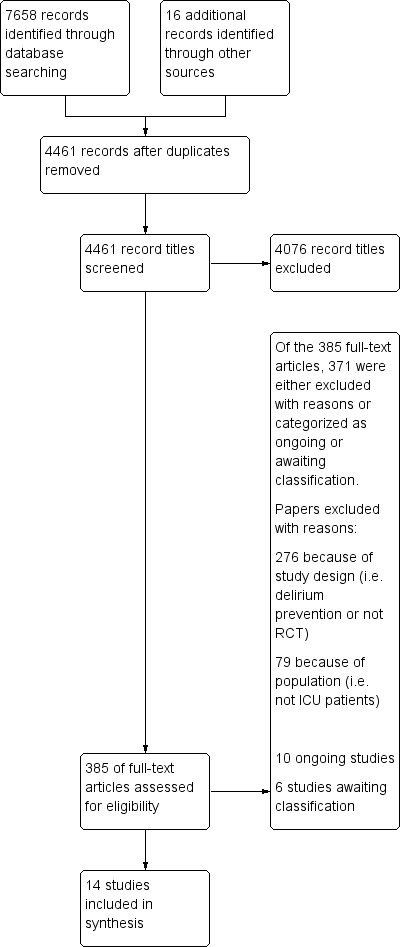
Study flow diagram.
Included studies
See the Characteristics of included studies table.
Study population
The 14 included studies recruited 1844 adult participants, with sample sizes ranging from 20 in Reade 2009 to 566 in Girard 2018. Seven studies enrolled more than 100 participants (Girard 2010a; Girard 2018; Hakim 2012; Needham 2016; Page 2013; Page 2017; van Eijk 2010). Twelve studies enrolled a mix of medical and surgical participants; two enrolled cardiovascular surgery participants only (Atalan 2013; Hakim 2012).
Eight studies used the CAM‐ICU to screen for delirium (Atalan 2013; Girard 2010a; Girard 2018; Needham 2016; Page 2013; Page 2017; Reade 2016; van Eijk 2010); the remaining six used the ICDSC (Al‐Qadheeb 2016; Bakri 2015; Devlin 2010; Hakim 2012; Reade 2009; Skrobik 2004). Five studies permitted inclusion of patients at high risk of developing delirium (i.e. delirium status not confirmed at study enrolment) (Al‐Qadheeb 2016; Girard 2010a; Hakim 2012; Page 2013; Reade 2009). Of these, two trials enrolled participants with subsyndromal delirium (Al‐Qadheeb 2016; Hakim 2012). One study enrolled mechanically ventilated participants with specifically agitated delirium (Reade 2009). Through written communication with the principal investigator, we confirmed that all participants had at a minimum subsyndromal delirium at enrolment, with 40% confirmed as delirious (i.e. ICDSC > 4). The remaining studies enrolled a combination of delirious and comatose participants (Girard 2010a; Page 2013), or investigators confirmed delirium status before enrolment (Girard 2018). These trials all examined delirium during ICU stay (and not thereafter).
Study design and setting
All trials but one were randomized (Skrobik 2004). Six trials were multi‐centre studies (Devlin 2010; Girard 2010a; Girard 2018; Needham 2016; Reade 2016; van Eijk 2010), and eight were single‐centre studies (Al‐Qadheeb 2016; Atalan 2013; Bakri 2015; Hakim 2012; Page 2013; Page 2017; Reade 2009; Skrobik 2004). Six studies were conducted in North America ‐ four exclusively in the USA (Al‐Qadheeb 2016; Girard 2010a; Girard 2018; Needham 2016), one exclusively in Canada (Skrobik 2004), and one in both Canada and the USA (Devlin 2010). The other studies took place in Australia and New Zealand (Reade 2009; Reade 2016), Egypt (Bakri 2015; Hakim 2012), the Netherlands (van Eijk 2010), Turkey (Atalan 2013), and the UK (Page 2013; Page 2017).
Interventions and comparators
Ten trials were placebo‐controlled (Al‐Qadheeb 2016; Devlin 2010; Girard 2010a; Girard 2018; Hakim 2012; Needham 2016; Page 2013; Page 2017; Reade 2016; van Eijk 2010). Four were head‐to‐head comparisons of different drugs (Atalan 2013; Bakri 2015; Reade 2009; Skrobik 2004). Three included three study groups (Bakri 2015; Girard 2010a; Girard 2018). Ten studied an antipsychotic intervention, predominantly haloperidol (Al‐Qadheeb 2016; Atalan 2013; Bakri 2015; Devlin 2010; Girard 2010a; Girard 2018; Hakim 2012; Page 2013; Reade 2009; Skrobik 2004). Three studied alpha2 agonists (all used dexmedetomidine) (Bakri 2015; Reade 2009; Reade 2016). Two trials studied a statin (Needham 2016; Page 2017). The remaining trials evaluated morphine (Atalan 2013), ondansetron (Bakri 2015), or rivastigmine (van Eijk 2010). Ten trials titrated the study drug based on symptoms or response (Atalan 2013; Bakri 2015; Devlin 2010; Girard 2010a; Girard 2018; Hakim 2012; Reade 2009; Reade 2016; Skrobik 2004; van Eijk 2010); four used fixed drug regimens (Al‐Qadheeb 2016; Needham 2016; Page 2013; Page 2017).
The extent to which study medication was given also varied, with some studies continuing drug for a fixed duration irrespective of whether delirium had resolved and others protocolizing discontinuation of the study drug once the patient was no longer delirious (Devlin 2010; Girard 2018; Page 2013). The duration of study drug exposure varied across trials including maximum of 28 days (Needham 2016; Page 2017), 14 days (Girard 2010a; Girard 2018; Page 2013), 10 days (Al‐Qadheeb 2016; Atalan 2013; Devlin 2010), seven days (Reade 2016), five days (Skrobik 2004), three days (Bakri 2015), as long as deemed medically necessary (Reade 2009), until delirium resolution or hospital discharge (van Eijk 2010), or for 24 hours after ICDSC was zero (Hakim 2012).
Eleven trials allowed use of an additional drug for management of breakthrough delirium symptoms or agitation (e.g. sedative, antipsychotic) (Atalan 2013; Bakri 2015; Devlin 2010; Girard 2010a; Girard 2018; Hakim 2012; Page 2013; Reade 2009; Reade 2016; Skrobik 2004; van Eijk 2010).
Outcomes
Outcomes varied in terms of measurement and reporting. All but two studies reported median (IQR) or mean (SD) for delirium duration (Bakri 2015; Skrobik 2004). The planned primary outcome defined as time from which delirium wasfirst identified to when it was first resolved was rarely reported (Devlin 2010). Most trials reported duration of delirium with variable definitions of resolved delirium (e.g. one negative score, two consecutive days with negative score, no definition provided). Therefore we chose to pool the results as duration of delirium as reported by study authors. Five studies reported median (IQR) or mean (SD) for number of days with coma (Girard 2010a; Girard 2018; Needham 2016; Page 2013; Page 2017); four reported median (IQR) or mean (SD) for number of days alive without delirium or coma (Girard 2010a; Girard 2018; Page 2013; Page 2017); eight reported median (IQR) or mean (SD) for mechanical ventilation duration (Al‐Qadheeb 2016; Atalan 2013; Devlin 2010; Girard 2010a; Girard 2018; Needham 2016; Reade 2009; Reade 2016); 11 reported median (IQR) or mean (SD) for ICU length of stay (Al‐Qadheeb 2016; Atalan 2013; Devlin 2010; Girard 2010a; Girard 2018; Hakim 2012; Needham 2016; Page 2013; Reade 2009; Reade 2016; van Eijk 2010); nine reported median (IQR) or mean (SD) for hospital length of stay (Atalan 2013; Devlin 2010; Girard 2018; Hakim 2012; Needham 2016; Page 2013; Page 2017; Reade 2016; van Eijk 2010); and 11 reported mortality at various time points (Al‐Qadheeb 2016; Atalan 2013; Devlin 2010; Girard 2010a; Girard 2018; Hakim 2012; Needham 2016; Page 2013; Page 2017; Reade 2009; van Eijk 2010). Three studies reported discharge disposition (Al‐Qadheeb 2016; Devlin 2010; Reade 2016). Reported adverse events included arrhythmias (Girard 2010a; Girard 2018; Page 2013; Reade 2009), extrapyramidal symptoms (Al‐Qadheeb 2016; Devlin 2010; Girard 2010a; Girard 2018; Hakim 2012; Page 2013; Skrobik 2004), use of physical restraints (Reade 2009; van Eijk 2010), unintentional device removal (Al‐Qadheeb 2016; Devlin 2010; Page 2013; Reade 2009; Reade 2016), and QTc prolongation (Al‐Qadheeb 2016; Bakri 2015; Devlin 2010; Girard 2010a; Girard 2018; Hakim 2012; Page 2013; Reade 2009).
For meta‐analysis and network meta‐analysis, we removed one open‐label trial from syntheses given what were judged to be special features in the study population (i.e. cardiovascular surgery, commonly associated with short ICU stays) and differences in baseline characteristics between dexmedetomidine and haloperidol arms despite randomization (Reade 2009).
Excluded studies
See Characteristics of excluded studies.
We excluded seven studies for the following reasons (Eremenko 2014; Khan 2019; Mailhot 2014; Pandharipande 2007; Riker 2009; Tagarakis 2012;Waszynski 2018): study design (Eremenko 2014; Pandharipande 2007; Riker 2009); no pharmacological intervention (Khan 2019; Mailhot 2014; Waszynski 2018); and no validated method to determine delirium (Tagarakis 2012).
Studies awaiting classification
See Characteristics of studies awaiting classification.
Six studies available as abstract ‐ Emerson 2014,Peters 2015, and Schoeffler 2012 ‐ or as trial registration ‐ NCT00429676,ISRCTN33122761, and NCT02366299 ‐ await classification due to insufficient information. These studies evaluate an antipsychotic (NCT00429676), clonidine (Schoeffler 2012), physostigmine (ISRCTN33122761), dexmedetomidine and propofol (NCT02366299), a multi‐component delirium management strategy (Emerson 2014), and intranasal insulin aspart (Peters 2015).
Ongoing studies
See Characteristics of ongoing studies.
Ten studies classified as ongoing studies will be monitored for incorporation into future updates of this review (IRCT20121231011956N10; IRCT20180911040998N1; NCT03392376; NCT01811459; NCT03317067; NCT02807467; NCT02216266; NCT02343575; NCT00351299; NCT03628391). Interventions include antipsychotics (NCT01811459; NCT03628391; IRCT20121231011956N10; IRCT20180911040998N1; NCT03392376), dexmedetomidine (NCT03317067; NCT02807467, NCT00351299), physostigmine (NCT02216266), and valproic acid (NCT02343575).
Risk of bias in included studies
We summarize risk of bias data in Figure 2 and Figure 3. Nine trials scored low risk of bias across all domains (Al‐Qadheeb 2016; Devlin 2010; Girard 2010a; Girard 2018; Needham 2016; Page 2013; Page 2017; Reade 2016; van Eijk 2010).
2.
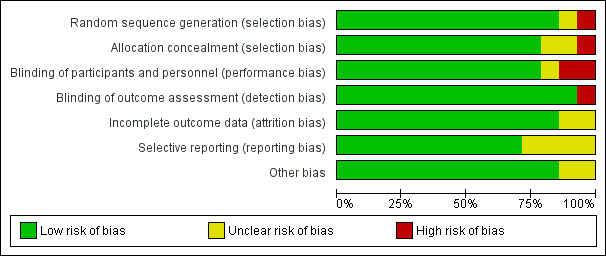
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.
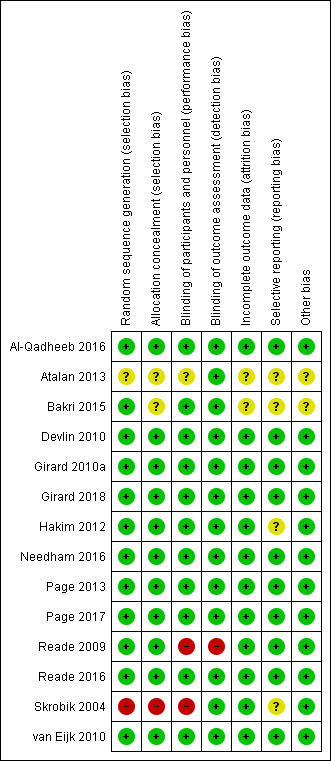
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
We judged all studies but two ‐ Atalan 2013 and Skrobik 2004 ‐ to have low risk of selection bias due to random sequence generation. Skrobik 2004 performed quasi‐randomization (i.e. even/odd enrolment day), and Atalan 2013 did not report the method of sequence generation. Twelve studies used computer‐generated randomization tables (Al‐Qadheeb 2016; Bakri 2015; Devlin 2010; Girard 2010a; Girard 2018; Hakim 2012; Page 2013; Needham 2016; Page 2017; Reade 2009; Reade 2016; van Eijk 2010). We judged eleven studies to have adequate allocation concealment via web‐based programs or sealed opaque envelopes (Al‐Qadheeb 2016; Devlin 2010; Girard 2010a; Girard 2018; Hakim 2012; Needham 2016; Page 2013; Page 2017; Reade 2009; Reade 2016; van Eijk 2010).
Blinding
Eleven studies have low risk of performance bias given blind design and explicit discussion of blinded study participants, clinicians, or study personnel (including outcome assessors) (Al‐Qadheeb 2016; Bakri 2015; Devlin 2010; Girard 2010a; Girard 2018; Hakim 2012; Needham 2016; Page 2013; Page 2017; Reade 2016; van Eijk 2010). We judged one study to have unclear risk of blinding bias as no details of blinding were available (Atalan 2013). We judged two studies to have high risk of bias as these trials lacked blinding (Reade 2009; Skrobik 2004). All trials but one had blinded outcome assessment (Reade 2009).
Incomplete outcome data
We judged all studies but two to have low risk of attrition bias as they accounted for all screened, enrolled, and randomized participants (Atalan 2013; Bakri 2015), and all except one employed an intention‐to‐treat principle in their analyses (Skrobik 2004). Two studies used a modified intention‐to‐treat analysis (e.g. modification permitted to account for post‐randomization circumstances that prevented use of data from certain participants) (Reade 2016; van Eijk 2010). We judged two studies to have unclear risk of attrition bias because they did not include figures, tables, or text outlining the numbers of participants who were screened, enrolled, and randomized, and/or who successfully completed the study protocol (Atalan 2013; Bakri 2015).
Selective reporting
We judged eleven studies to have low risk of reporting bias based on examination of their respective trial registration or published protocols (Al‐Qadheeb 2016; Devlin 2010; Girard 2010a; Girard 2018; Hakim 2012; Needham 2016; Page 2013; Page 2017; Reade 2009; Reade 2016; van Eijk 2010). The remaining trials were deemed at unclear risk, as trial registrations or protocols were not available to confirm outcome reporting.
Other potential sources of bias
We judged all studies but two to have low risk of other potential sources of bias (Atalan 2013; Bakri 2015). All studies cited funding sources, except Atalan 2013, which provided no funding details. Two studies were conducted without external funding (Bakri 2015; Hakim 2012). Study support for a pharmaceutical company was declared in seven studies; however all stated that these companies had no involvement in study design, data collection, analysis, or data reporting (Devlin 2010; Girard 2010a; Needham 2016; Reade 2009; Reade 2016; Skrobik 2004; van Eijk 2010).
Effects of interventions
See: Table 1; Table 2; Table 3; Table 4; Table 5; Table 6
See Summary of findings tables (Table 1; Table 2; Table 3; Table 4; Table 5; Table 6). The 'Summary of findings' tables provide overall estimates of treatment effects compared with placebo. We summarize the quality of evidence for delirium duration, delirium‐free and coma‐free days, duration of mechanical ventilation, and length of ICU stay obtained through pairwise comparisons and NMA.
Geometry of evidence networks by endpoints
For all outcomes, most trials compared one active intervention (drug) against placebo; trials involving comparisons between active interventions were rare. The number of participants enrolled for each active therapy was small in the networks compared to the number enrolled for placebo comparisons. Figure 4 (panel A to F) presents the network diagrams that indicate corresponding eligible regimens in the evidence network for each outcome. Each line links treatments directly compared across studies. Head‐to‐head trials were available for 7/21 (33%) of the pairwise comparisons for delirium duration, 6/15 (40%) for duration of mechanical ventilation, 7/21 (33%) for hospital length of stay, 7/21 (33%) for ICU length of stay, 4/6 (67%) for delirium‐free and coma‐free days, and 4/6 (67%) for days in coma.
4.
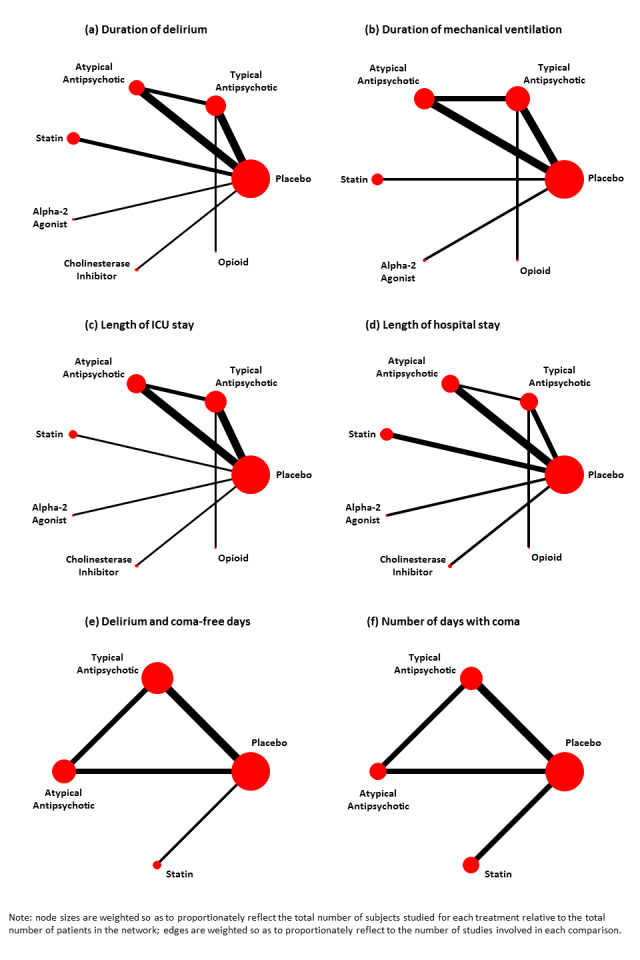
Network diagrams of pairwise comparisons for the six outcomes with network meta‐analyses.
Primary outcome
1. Duration of delirium
Pairwise meta‐analysis (direct comparisons)
Data from a total of 11 trials (n = 1530 participants) contributed to the analysis of duration of delirium (Al‐Qadheeb 2016; Atalan 2013; Devlin 2010; Girard 2010a; Girard 2018; Hakim 2012; Needham 2016; Page 2013; Page 2017; Reade 2016; van Eijk 2010); ten RCTs (n = 1477 participants) were placebo‐controlled. Treatment effect estimates from pairwise meta‐analyses are reported in Analysis 1.1 and Figure 5. Pairwise meta‐analyses showed that the alpha2 agonist dexmedetomidine may be associated with a shorter duration of delirium (ratio of means (RoM) 0.58, 95% confidence interval (CI) 0.43 to 0.79; 71 participants; 1 study), and the cholinesterase inhibitor rivastigmine may be associated with a longer duration of delirium (RoM 1.84, 95% CI 1.25 to 2.69; 104 participants; 1 study) compared to placebo. The pairwise meta‐analyses showed no effect on the duration of delirium for typical antipsychotics (RoM 1.02, 95% CI 0.91 to 1.14; 608 participants; 4 studies), atypical antipsychotics (RoM 0.73, 95% CI 0.49 to 1.11; 500 participants; 4 studies), or statins (RoM 1.07, 95% CI 0.91 to 1.25; 414 participants; 2 studies) compared to placebo.
1.1. Analysis.
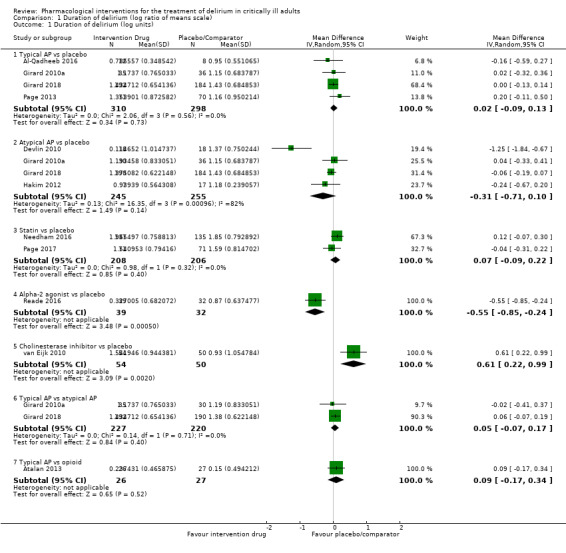
Comparison 1 Duration of delirium (log ratio of means scale), Outcome 1 Duration of delirium (log units).
5.
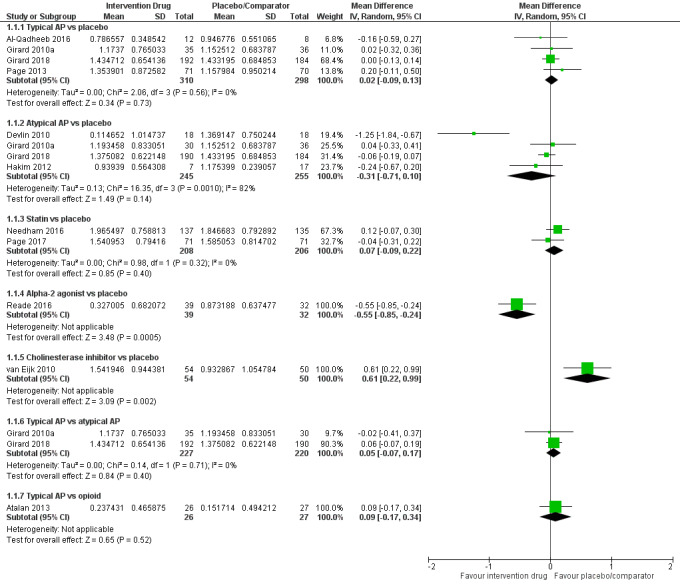
Forest plot of comparison: 1 Duration of delirium (log units), outcome: 1.1 Duration of delirium (log units).
NMA (combinations of direct and indirect comparisons)
The random‐effects consistency model was an adequate fit, with posterior total residual deviance of 27.33 (compared to 24 unconstrained data points). The forest plot in Figure 6 presents the ratio of means (RoM) estimates for each intervention compared to placebo derived from the random‐effects consistency model, along with 95% credible intervals (CrIs). For all interventions compared to placebo, 95% CrIs were wide and failed to rule out the possibility of no difference. The intervention with the smallest RoM (i.e. most preferred) was the alpha2 agonist dexmedetomidine (RoM 0.58, 95% CrI 0.26 to 1.27; SUCRA 0.895; moderate‐quality evidence) (Table 7). In order of descending surface under the cumulative ranking curve (SUCRA) values (best to worst; Table 8), the next best interventions were atypical antipsychotics (RoM vs placebo 0.80, 95% CrI 0.50 to 1.11; SUCRA 0.738; moderate‐quality evidence), opioids (RoM vs placebo 0.88, 95% CrI 0.37 to 2.01; SUCRA 0.578; very low‐quality evidence), typical antipsychotics (RoM vs placebo 0.96, 95% CrI 0.64 to 1.36; SUCRA 0.468; high‐quality evidence), placebo (SUCRA 0.403), statins (RoM vs placebo 1.05, 95% CrI 0.61 to 1.77; SUCRA 0.365; moderate‐quality evidence), and the cholinesterase inhibitor rivastigmine (RoM vs placebo 1.84, 95% CrI 0.82 to 4.10; SUCRA 0.054; moderate‐quality evidence). In addition to comparisons versus placebo, Table 7 shows the comparisons between active interventions. As an example of interpretation, the RoM estimate of 0.58 (95% CrI 0.26 to 1.27) in the lower triangle suggests a 42% reduction in the mean duration of delirium with alpha2 agonists compared to placebo. The corresponding probability estimate in the upper triangle suggests a probability of 93.8% that alpha2 agonists are better than placebo in terms of duration of delirium. The between‐study SD, as a measure of heterogeneity, was estimated to be 0.29 (95% CrI 0.03 to 0.75). Comparison of DIC values between the random‐effects consistency model (‐14.66) and the corresponding random‐effects unrelated means model (DIC ‐14.55), as well as inspection of a scatterplot of posterior mean deviance contributions from both models (Figure 7), suggested no violation of the consistency assumption. Our inspection of Gelman‐Rubin‐Brooks diagnostics and potential scale reduction factors for all NMAs confirmed convergence with 200,000 iterations in all cases (among which 100,000 were burn‐in).
6.
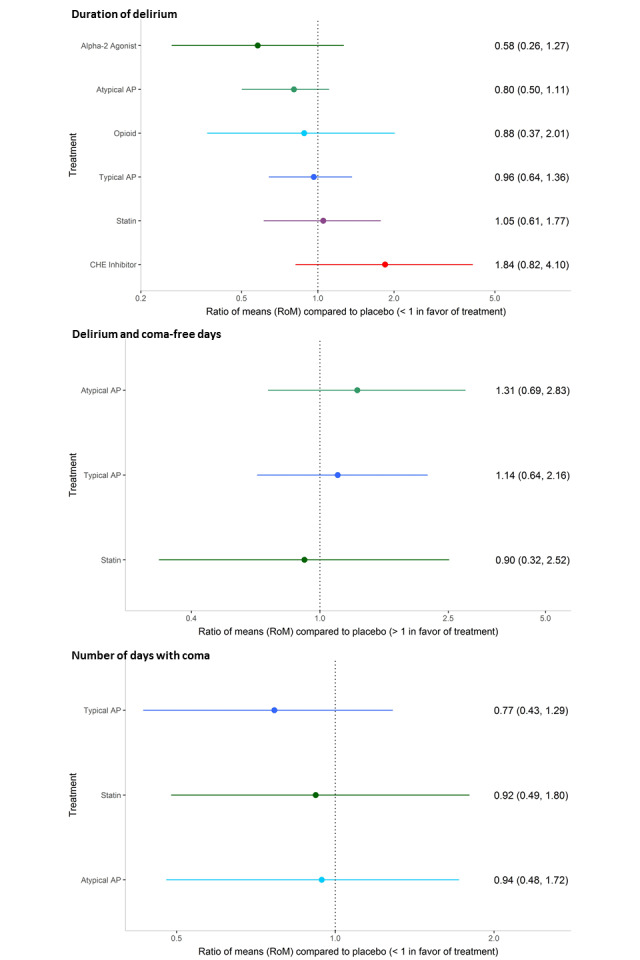
Findings from network meta‐analysis: duration of delirium, delirium‐free and coma‐free days, and days with coma.
1. Duration of delirium: league table of posterior median pairwise RoM and 95% CrI (lower triangle), and pairwise probabilities that a treatment is better than another (upper triangle).
| Alpha2 agonist | 0.807 | 0.819 | 0.907 | 0.922 | 0.977 | 0.938 |
| 0.72 (0.33, 1.87) |
Atypical antipsychotic |
0.610 | 0.858 | 0.861 | 0.975 | 0.927 |
| 0.66 (0.21, 2.13) | 0.92 (0.36, 2.11) | Opioid | 0.627 | 0.685 | 0.924 | 0.660 |
| 0.60 (0.26, 1.48) | 0.84 (0.50, 1.25) | 0.92 (0.42, 1.97) |
Typical antipsychotic |
0.646 | 0.945 | 0.607 |
| 0.55 (0.22, 1.43) | 0.77 (0.37, 1.38) | 0.84 (0.30, 2.25) | 0.92 (0.47, 1.73) | Statin | 0.909 | 0.396 |
| 0.31 (0.10, 0.97) | 0.44 (0.17, 1.00) | 0.48 (0.14, 1.51) | 0.52 (0.21, 1.25) | 0.57 (0.22, 1.49) |
CHE Inhibitor |
0.054 |
| 0.58 (0.26, 1.27) | 0.80 (0.50, 1.11) | 0.88 (0.37, 2.01) | 0.96 (0.64, 1.36) | 1.05 (0.61, 1.77) | 1.84 (0.82, 4.10) | Placebo |
CHE: cholinesterase.
Crl: credible interval.
RoM: ratio of means.
A complete summary of estimates for efficacy from the random‐effects (RE) consistency model assuming vague priors is displayed.
Treatments other than placebo are in the order of decreasing surface under the cumulative ranking curve (SUCRA) value from upper left to lower right. For each comparison, the lower/right‐most treatment is the reference treatment. For example, the RoM estimate of 0.58 (95% CrI 0.26 to 1.27) in the lower triangle suggests a 42% reduction in the mean duration of delirium with alpha2 agonists compared to placebo. The corresponding probability estimate in the upper triangle suggests a probability of 93.8% that alpha2 agonists are better than placebo in terms of duration of delirium. Estimates which ruled out the possibility of no difference based on pairwise RoM estimates are shown in bold font.
2. Duration of delirium: mean SUCRA value, mean probability to be the best, and mean rank for each treatment.
| RE consistency model | |||
| Mean SUCRA | Mean Pr(best) | Mean ranka | |
| Alpha2 agonist | 0.895 | 0.717 | 1.63 (1 to 6) |
| Atypical antipsychotic | 0.738 | 0.114 | 2.57 (1 to 5) |
| Opioid | 0.578 | 0.129 | 3.53 (1 to 7) |
| Typical antipsychotic | 0.468 | 0.010 | 4.19 (2 to 6) |
| Placebo | 0.403 | 0.001 | 4.58 (3 to 6) |
| Statin | 0.365 | 0.023 | 4.81 (2 to 7) |
| CHE inhibitor | 0.054 | 0.006 | 6.68 (3 to 7) |
aMean rank with 2.5% and 97.5% quantiles in parentheses.
CHE: cholinesterase.
Pr: probability.
RE: random‐effects.
SUCRA: surface under the cumulative ranking curve.
7.
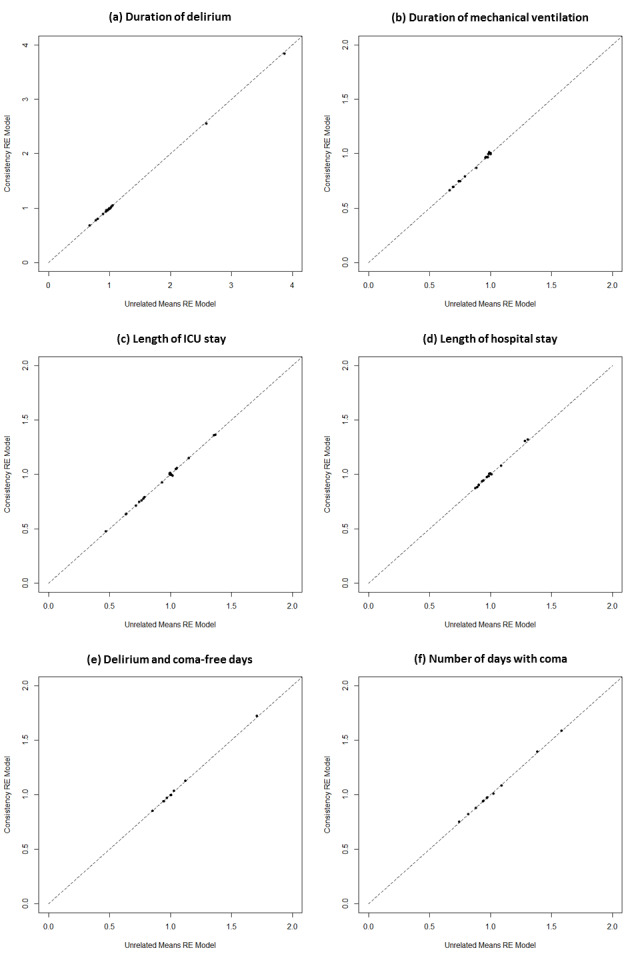
Consistency assumption check: posterior mean deviance contribution plots for RE consistency model vs unrelated means model; they did not suggest violation of the consistency assumption.
Secondary outcomes
1. a) Delirium‐free and coma‐free days
Pairwise meta‐analysis (direct comparisons)
Data from a total of four trials (n = 950 participants) contributed to the analysis of delirium‐free and coma‐free days (Girard 2010a; Girard 2018; Page 2013; Page 2017). Studies were placebo‐controlled with antipsychotics ‐ in Girard 2010a,Girard 2018, and Page 2013 ‐ or statins ‐ in Page 2017 ‐ as the intervention. No pairwise comparison resulted in fewer delirium‐free and coma‐free days (Analysis 2.1; Figure 8).
2.1. Analysis.
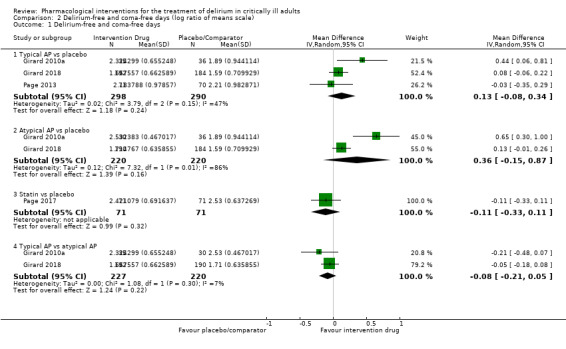
Comparison 2 Delirium‐free and coma‐free days (log ratio of means scale), Outcome 1 Delirium‐free and coma‐free days.
8.
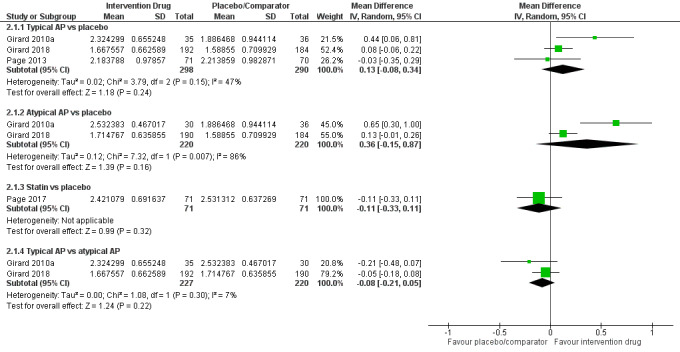
Forest plot of comparison: 2 Delirium‐free and coma‐free days (log units), outcome: 2.1 Delirium‐free and coma‐free days.
NMA (combinations of direct and indirect comparisons)
The forest plot in Figure 6 presents estimates for all interventions compared to placebo from the random‐effects consistency model. The random‐effects consistency model was an adequate fit, with posterior total residual deviance of 10.59 (compared to 10 unconstrained data points). The intervention with the largest RoM was atypical antipsychotics (RoM vs placebo 1.31, 95% CrI 0.69 to 2.83; SUCRA 0.845; moderate‐quality evidence). In order of descending SUCRA values, the next best interventions were typical antipsychotics (RoM vs placebo 1.14, 95% CrI 0.64 to 2.16; SUCRA 0.589; moderate‐quality evidence), placebo (SUCRA 0.327), and statins (RoM vs placebo 0.90, 95% CrI 0.32 to 2.52; SUCRA 0.239; moderate‐quality evidence). In addition to comparisons versus placebo, comparisons between active interventions are provided in Table 9, and secondary measures of effect are presented in Table 10. In all cases, 95% CrIs were wide and failed to rule out the possibility of no difference. The between‐study SD, as a measure of heterogeneity, was estimated to be 0.37 (95% CrI 0.02 to 1.42). Comparison of DIC values between the random‐effects consistency model (‐11.23) and the corresponding random‐effects unrelated means model (DIC ‐11.26), as well as inspection of a scatterplot of posterior mean deviance contributions from both models (Figure 7), did not suggest violation of the consistency assumption.
3. Delirium‐ and coma‐free days: league table of posterior median pairwise RoM and 95% (lower triangle), and pairwise probabilities that a treatment is better than another (upper triangle).
| Atypical antipsychotic | 0.784 | 0.854 | 0.898 |
| 1.15 (0.58, 2.40) |
Typical antipsychotic |
0.768 | 0.781 |
| 1.46 (0.45, 5.41) | 1.27 (0.40, 4.30) | Statin | 0.340 |
| 1.31 (0.69, 2.83) | 1.14 (0.64, 2.16) | 0.90 (0.32, 2.52) | Placebo |
RoM: ratio of means.
A complete summary of estimates for efficacy from the random‐effects (RE) consistency model assuming vague priors is displayed.
Treatments other than placebo are in the order of decreasing surface under the cumulative ranking curve (SUCRA) value from upper left to lower right. For each comparison, the lower/right‐most treatment is the reference treatment. For example, the RoM estimate of 1.31 (95% credible interval (CrI) 0.69 to 2.83) in the lower triangle suggests a 31% increase in mean delirium‐ and coma‐free days with atypical antipsychotics compared to placebo. The corresponding probability estimate in the upper triangle suggests a probability of 89.8% that atypical antipsychotics are better than placebo in terms of delirium‐ and coma‐free days.
4. Delirium‐ and coma‐free days: mean SUCRA value, mean probability to be the best, and mean rank for each treatment.
| RE consistency model | |||
| Mean SUCRA | Mean Pr(best) | Mean ranka | |
| Atypical antipsychotic | 0.845 | 0.690 | 1.46 (1 to 4) |
| Typical antipsychotic | 0.589 | 0.160 | 2.23 (1 to 4) |
| Placebo | 0.327 | 0.033 | 3.02 (1 to 4) |
| Statin | 0.239 | 0.116 | 3.28 (1 to 4) |
aMean rank with 2.5% and 97.5% quantiles in parentheses.
Pr: probability.
RE: random‐effects.
SUCRA: surface under the cumulative ranking curve.
1. b) Days with coma
Pairwise meta‐analysis (direct comparisons)
Data from a total of five trials (n = 1222 participants) contributed to the analysis of days with coma (Girard 2010a; Girard 2018; Needham 2016; Page 2013; Page 2017). All five studies were placebo‐controlled, with antipsychotics (in Girard 2010a,Girard 2018, and Page 2013) or statins (in Needham 2016 and Page 2017) as the intervention group. No pairwise comparison resulted in fewer days with coma (Analysis 3.1; Figure 9).
3.1. Analysis.
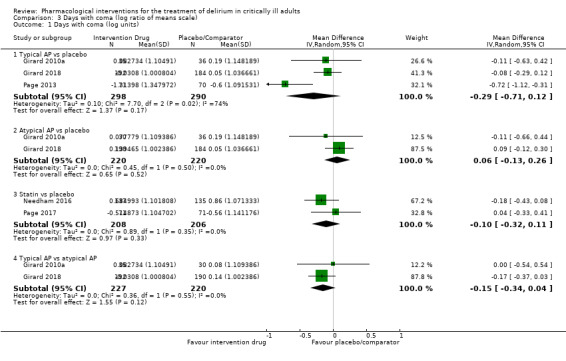
Comparison 3 Days with coma (log ratio of means scale), Outcome 1 Days with coma (log units).
9.
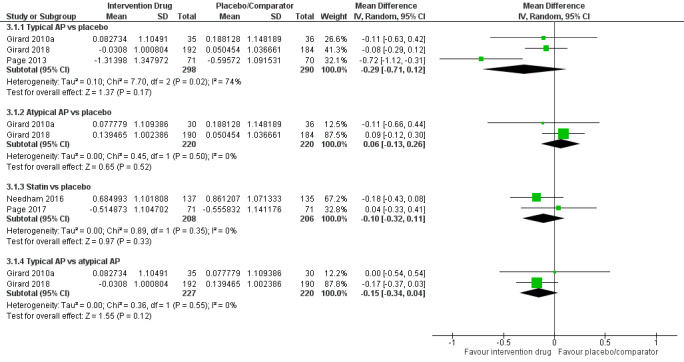
Forest plot of comparison: 3 Days with coma (log units), outcome: 3.1 Days with coma (log units).
NMA (combinations of direct and indirect comparisons)
The forest plot in Figure 6 presents estimates for each intervention compared to placebo from the random‐effects consistency model. The random‐effects consistency model was an adequate fit, with a posterior total residual deviance of 12.34 (compared to 12 unconstrained data points). The intervention with the smallest RoM versus placebo was typical antipsychotics (RoM vs placebo 0.77, 95% CrI 0.43 to 1.29; SUCRA 0.820; low‐quality evidence). In order of descending SUCRA values, the next best interventions were statins (RoM vs placebo 0.92, 95% CrI 0.49 to 1.80; SUCRA 0.481; moderate‐quality evidence), atypical antipsychotics (RoM vs placebo 0.94, 95% CrI 0.48 to 1.72; SUCRA 0.422; moderate‐quality evidence), and placebo (SUCRA 0.278). In addition to comparisons versus placebo, comparisons between active interventions are provided in Table 11, and secondary measures of effect are presented in Table 12. In all cases, 95% CrIs were wide and failed to rule out the possibility of no difference. The between‐study SD, as a measure of heterogeneity, was estimated to be 0.34 (95% CrI 0.03 to 1.08). Comparison of DIC values between the random‐effects consistency model (‐5.32) and the corresponding random‐effects unrelated means model (‐5.33), as well as inspection of a scatterplot of posterior mean deviance contributions from both models (Figure 7), did not suggest violation of the consistency assumption.
5. Days with coma: league table of posterior median pairwise RoM and 95% CrI (lower triangle), and pairwise probabilities that a treatment is better than another (upper triangle).
|
Typical antipsychotic |
0.740 | 0.815 | 0.905 |
| 0.83 (0.34, 1.87) | Statin | 0.532 | 0.651 |
| 0.81 (0.44, 1.56) | 0.98 (0.41, 2.58) |
Atypical antipsychotic |
0.612 |
| 0.77 (0.43, 1.29) | 0.92 (0.49, 1.80) | 0.94 (0.48, 1.72) | Placebo |
Crl: credible interval.
RoM: ratio of means.
A complete summary of estimates for efficacy from the random‐effects (RE) consistency model assuming vague priors is displayed.
Treatments other than placebo are in the order of decreasing surface under the cumulative ranking curve (SUCRA) value from upper left to lower right. For each comparison, the lower/right‐most treatment is the reference treatment. For example, the RoM estimate of 0.77 (95% CrI 0.43 to 1.29) in the lower triangle suggests a 23% reduction in mean coma days with typical antipsychotics compared to placebo. The corresponding probability estimate in the upper triangle suggests a probability of 90.5% that typical antipsychotics are better than placebo in terms of days with coma.
6. Days with coma: mean SUCRA values, mean probability to be the best, and mean rank for each treatment.
| RE consistency model | |||
| Mean SUCRA | Mean Pr(best) | Mean ranka | |
| Typical antipsychotic | 0.820 | 0.620 | 1.54 (1 to 4) |
| Statin | 0.481 | 0.222 | 2.56 (1 to 4) |
| Atypical antipsychotic | 0.422 | 0.132 | 2.73 (1 to 4) |
| Placebo | 0.278 | 0.026 | 3.17 (1 to 4) |
aMean rank with 2.5% and 97.5% quantiles in parentheses.
Pr: probability.
RE: random‐effects.
SUCRA: surface under the cumulative ranking curve
2. Relapse of delirium (% patients)
No study reported data on this outcome.
3. Resolution of delirium symptoms (e.g. hallucinations, agitation)
No study reported data on resolution of delirium symptoms as a specific outcome. Agitation was reported in three studies (Al‐Qadheeb 2016; Devlin 2010; Page 2013). Al‐Qadheeb 2016 found that the haloperidol group spent fewer hours per study day agitated (Sedation Agitation Scale ≥ 5) compared to the placebo group (median 0 vs 2; P = 0.008). Similarly, Devlin 2010 found that quetiapine was associated with fewer hours of agitation (SAS ≥ 5) compared to placebo (6 vs 36; P = 0.02). Page 2013 found that a smaller proportion of participants had agitated Richmond Agitation‐Sedation Scale (RASS) scores (RASS > 2+) in the first 14 days of the study in the haloperidol group compared to the placebo group (median 13% vs 20%; P = 0.0075).
4. Duration of mechanical ventilation
Pairwise meta‐analysis (direct comparisons)
Data from a total of seven trials (n = 1167 participants) contributed to the analysis of duration of mechanical ventilation (Al‐Qadheeb 2016; Atalan 2013; Devlin 2010; Girard 2010a; Girard 2018; Needham 2016; Reade 2016); all but one study was placebo‐controlled (Atalan 2013). Trials evaluated dexmedetomidine (Reade 2016), antipsychotics (Al‐Qadheeb 2016, Devlin 2010; Girard 2010a; Girard 2018), opioids (Atalan 2013), and statins (Needham 2016). For meta‐analysis and network meta‐analysis, we could not include Page 2013 or Reade 2009 in the syntheses of mechanical ventilation duration. We excluded Page 2013 due to missing SD. We excluded Reade 2009 as the addition of this trial resulted in problems with the consistency equation. We judged there to be important differences in study populations (i.e. cardiovascular surgery commonly associated with short ICU stays) that explained the disruption of the consistency equation. Amongst the pairwise comparisons versus placebo (Analysis 4.1; Figure 10), dexmedetomidine was associated with a reduced duration of mechanical ventilation (RoM 0.55, 95% CI 0.41 to 0.75; 71 participants; 1 study), and typical antipsychotics (RoM 0.92, 95% CI 0.79 to 1.06; 515 participants; 3 studies), atypical antipsychotics (RoM 0.98, 95% CI 0.84 to 1.34; 476 participants; 3 studies), and statins (RoM 1.09, 95% CI 0.90 to 1.34; 272 participants; 1 study) did not.
4.1. Analysis.
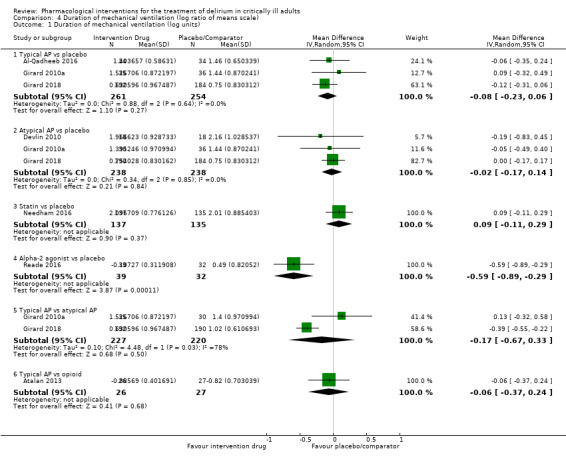
Comparison 4 Duration of mechanical ventilation (log ratio of means scale), Outcome 1 Duration of mechanical ventilation (log units).
10.
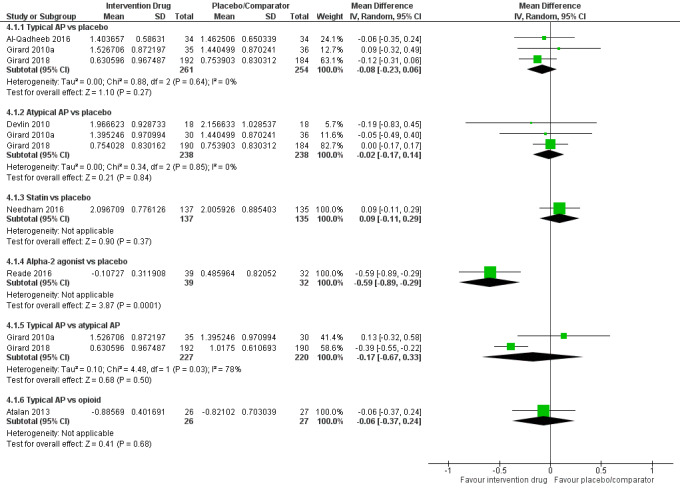
Forest plot of comparison: 4 Duration of mechanical ventilation (log units), outcome: 4.1 Duration of mechanical ventilation (log units).
NMA (combinations of direct and indirect comparisons)
The forest plot in Figure 11 presents RoM estimates for each intervention compared to placebo from the random‐effects consistency model. The random‐effects consistency model was an adequate fit, with posterior total residual deviance of 14.13 (compared to 16 unconstrained data points). The intervention with the smallest RoM versus placebo was dexmedetomidine (RoM 0.55, 95% CrI 0.34 to 0.89; SUCRA 0.974; moderate‐quality evidence). In order of descending SUCRA values, the next best interventions were typical antipsychotics (RoM 0.93, 95% CI 0.72 to 1.24; SUCRA 0.576; moderate‐quality evidence), atypical antipsychotics (RoM 0.98, 95% CI 0.71 to 1.28; SUCRA 0.440; moderate‐quality evidence), opioids (RoM vs placebo 0.99, 95% CrI 0.58 to 1.76; SUCRA 0.410; very low‐quality evidence), placebo (SUCRA 0.377), and statins (RoM vs placebo 1.10, 95% CrI 0.71 to 1.69; SUCRA 0.223; moderate‐quality evidence). Comparisons between active interventions are provided in Table 13, and secondary measures of effect are presented in Table 14. The between‐study SD, as a measure of heterogeneity, was estimated to be 0.14 (95% CrI 0.005 to 0.53). Comparison of DIC values between the random‐effects consistency model (‐15.16) and the corresponding random‐effects unrelated means model (‐15.24), as well as inspection of a scatterplot of posterior mean deviance contributions from both models (Figure 7), did not suggest violation of the consistency assumption.
11.
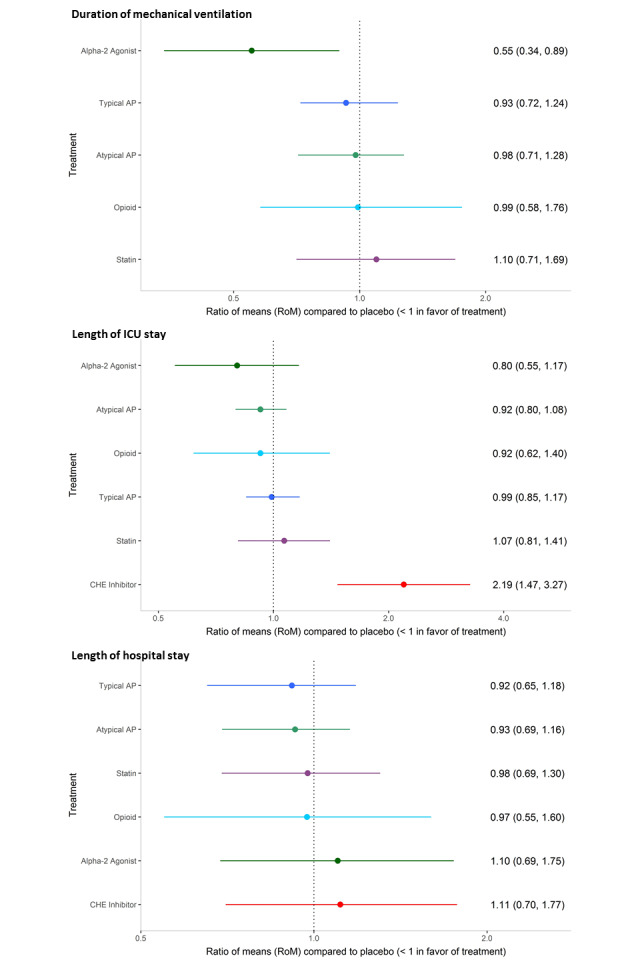
Findings from network meta‐analysis: duration of mechanical ventilation, length of ICU and hospital stay.
7. Duration of mechanical ventilation: league table of posterior median pairwise RoM and 95% CrI (lower triangle), and pairwise probabilities that a treatment is better than another (upper triangle).
| Alpha2 agonist | 0.973 | 0.973 | 0.958 | 0.978 | 0.986 |
| 0.59 (0.34, 1.01) |
Typical antipsychotic |
0.665 | 0.628 | 0.805 | 0.754 |
| 0.57 (0.33, 1.02) | 0.95 (0.72, 1.35) |
Atypical antipsychotic |
0.527 | 0.729 | 0.582 |
| 0.56 (0.26, 1.14) | 0.94 (0.58, 1.52) | 0.98 (0.53, 1.70) | Opioid | 0.645 | 0.517 |
| 0.50 (0.26, 0.97) | 0.85 (0.52, 1.45) | 0.89 (0.52, 1.47) | 0.90 (0.46, 1.87) | Statin | 0.274 |
| 0.55 (0.34, 0.89) | 0.93 (0.72, 1.24) | 0.98 (0.71, 1.28) | 0.99 (0.58, 1.76) | 1.10 (0.71, 1.69) | Placebo |
Crl: credible interval.
RoM: ratio of means.
A complete summary of estimates for efficacy from the random‐effects (RE) consistency model assuming vague priors is displayed.
Treatments other than placebo are in the order of decreasing surface under the cumulative ranking curve (SUCRA) value from upper left to lower right. For each comparison, the lower/right‐most treatment is the reference treatment. For example, the RoM estimate of 0.55 (95% CrI 0.34 to 0.89) in the lower triangle suggests a 45% reduction in the mean duration of mechanical ventilation with alpha2 agonists compared to placebo. The corresponding probability estimate in the upper triangle suggests a probability of 98.6% that alpha2 agonists are better than placebo for the duration of mechanical ventilation. Estimates which ruled out the possibility of no difference based on pairwise RoM estimates are shown in bold font.
8. Duration of mechanical ventilation: mean SUCRA values, mean probability to be the best, and mean rank for each treatment.
| RE consistency model | |||
| Mean SUCRA | Mean Pr(best) | Mean ranka | |
| Alpha2 agonists | 0.974 | 0.931 | 1.13 (1 to 3) |
| Typical antipsychotic | 0.576 | 0.009 | 3.12 (2 to 6) |
| Atypical antipsychotic | 0.440 | 0.012 | 3.80 (2 to 6) |
| Opioid | 0.410 | 0.033 | 3.95 (1 to 6) |
| Placebo | 0.377 | 0.001 | 4.11 (2 to 6) |
| Statin | 0.223 | 0.014 | 4.88 (2 to 6) |
aMean rank with 2.5% and 97.5% quantiles in parentheses.
Pr: probability.
RE: random‐effects.
SUCRA: surface under the cumulative ranking curve.
5. a) Length of ICU stay
Pairwise meta‐analysis (direct comparisons)
Data from a total of 10 trials (n = 1475 participants) contributed to the analysis of length of ICU stay (Al‐Qadheeb 2016; Atalan 2013; Devlin 2010; Girard 2010a; Girard 2018; Hakim 2012; Needham 2016; Page 2013; Reade 2016; van Eijk 2010); all but one trial were placebo‐controlled (Atalan 2013). Atypical antipsychotics were associated with significantly reduced length of ICU stay (RoM 0.91, 95% CI 0.84 to 1.00; 577 participants; 4 studies), and the cholinesterase inhibitor rivastigmine was associated with significantly increased length of ICU stay (RoM 2.18, 95% CI 1.58 to 3.03; 104 participants; 1 study) compared to placebo (Analysis 5.1;Figure 12). No difference was found for typical antipsychotics (RoM 1.01, 95% CI 0.90 to 1.14; 618 participants; 4 studies), statins (RoM 1.06, 95% CI 0.91 to 1.23; 272 participants; 1 study), or alpha2 agonists (RoM 0.80, 95% CI 0.59 to 1.08; 71 participants; 1 study) compared to placebo.
5.1. Analysis.
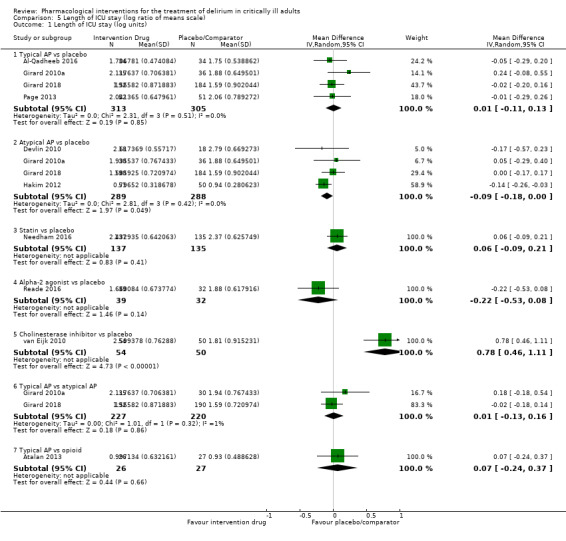
Comparison 5 Length of ICU stay (log ratio of means scale), Outcome 1 Length of ICU stay (log units).
12.
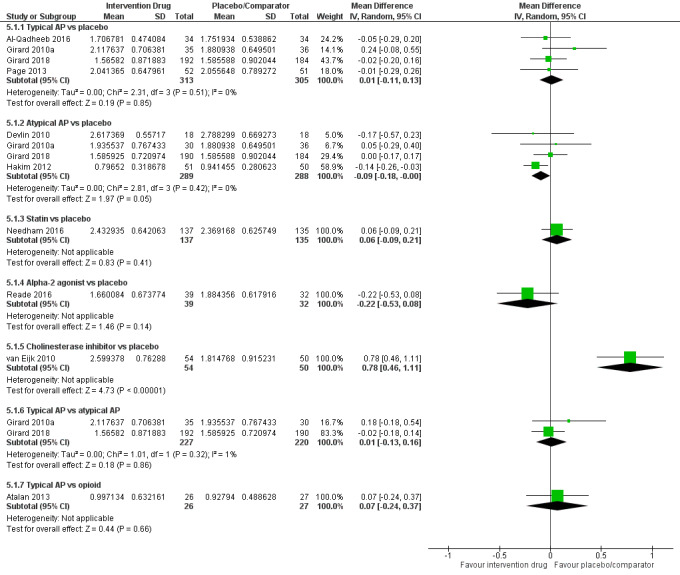
Forest plot of comparison: 5 Length of ICU stay (log units), outcome: 5.1 Length of ICU stay (log units).
NMA (combinations of direct and indirect comparisons)
The forest plot in Figure 11 presents RoM estimates for each intervention compared to placebo from the random‐effects consistency model. The random‐effects consistency model was an adequate fit, with posterior total residual deviance of 20.45 (compared to 22 unconstrained data points). The cholinesterase inhibitor rivastigmine was found to have longer length of ICU stay compared to placebo, and all remaining comparisons showed wide 95% CrIs that failed to rule out the possibility of no difference. The intervention with the smallest RoM versus placebo was dexmedetomidine (RoM 0.80, 95% CrI 0.55 to 1.17; SUCRA 0.853; low‐quality evidence). In order of descending SUCRA values, the next best interventions were atypical antipsychotics (RoM vs placebo 0.92, 95% CrI 0.80 to 1.08; SUCRA 0.709; high‐quality evidence), opioids (RoM vs placebo 0.92, 95% CrI 0.62 to 1.40; SUCRA 0.639; very low‐quality evidence), typical antipsychotics (RoM vs placebo 0.99, 95% CrI 0.85 to 1.17; SUCRA 0.496; moderate‐quality evidence), placebo (SUCRA 0.457), statins (RoM vs placebo 1.07, 95% CrI 0.81 to 1.41; SUCRA 0.344; low‐quality evidence), and cholinesterase inhibitors (RoM vs placebo 2.19, 95% CrI 1.47 to 3.27; SUCRA 0.002; moderate‐quality evidence). Comparisons between active interventions are provided in Table 15, and secondary measures of effect are presented in Table 16. The between‐study SD, as a measure of heterogeneity, was estimated to be 0.09 (95% CrI 0.003 to 0.28). Comparison of DIC values between the random‐effects consistency model (‐27.94) and the corresponding random‐effects unrelated means model (‐27.97), as well as inspection of a scatterplot of posterior mean deviance contributions from both models (Figure 7), did not suggest violation of the consistency assumption.
9. Length of ICU stay: league table of posterior median pairwise RoM and 95% CrI (lower triangle), and pairwise probabilities that a treatment is better than another (upper triangle).
| Alpha2 agonist | 0.766 | 0.705 | 0.858 | 0.902 | 0.999 | 0.886 |
| 0.87 (0.58, 1.29) |
Atypical antipsychotic |
0.499 | 0.792 | 0.856 | 0.999 | 0.874 |
| 0.87 (0.50, 1.49) | 1.00 (0.65, 1.51) | Opioid | 0.647 | 0.736 | 0.996 | 0.658 |
| 0.81 (0.54, 1.21) | 0.93 (0.77, 1.12) | 0.93 (0.64, 1.36) |
Typical antipsychotic |
0.714 | 0.998 | 0.559 |
| 0.75 (0.47, 1.20) | 0.87 (0.64, 1.20) | 0.87 (0.53, 1.44) | 0.93 (0.68, 1.29) | Statin | 0.995 | 0.278 |
| 0.37 (0.21, 0.63) | 0.42 (0.28, 0.65) | 0.42 (0.24, 0.75) | 0.45 (0.30, 0.70) | 0.49 (0.30, 0.79) | CHE inhibitor | 0.001 |
| 0.80 (0.55, 1.17) | 0.92 (0.80, 1.08) | 0.92 (0.62, 1.40) | 0.99 (0.85, 1.17) | 1.07 (0.81, 1.41) | 2.19 (1.47, 3.27) | Placebo |
CHE: cholinesterase.
Crl: credible interval.
ICU: intensive care unit.
RoM: ratio of means.
A complete summary of estimates for efficacy from the random‐effects (RE) consistency model assuming vague priors is displayed.
Treatments other than placebo are in the order of decreasing surface under the cumulative ranking curve (SUCRA) value from upper left to lower right. For each comparison, the lower/right‐most treatment is the reference treatment. For example, the RoM estimate of 0.80 (95% CrI 0.55 to 1.17) in the lower triangle suggests a 20% reduction in mean length of ICU stay with alpha2 agonists compared to placebo. The corresponding probability estimate in the upper triangle suggests a probability of 88.6% that alpha2 agonists are better than placebo for the length of ICU stay. Estimates which ruled out the possibility of no difference based on pairwise RoM estimates are shown in bold font.
10. Length of ICU stay: mean SUCRA value, mean probability to be the best, and mean rank for each treatment.
| RE consistency model | |||
| Mean SUCRA | Mean Pr(best) | Mean ranka | |
| Alpha2 agonists | 0.853 | 0.608 | 1.88 (1 to 6) |
| Atypical antipsychotic | 0.709 | 0.106 | 2.75 (1 to 5) |
| Opioid | 0.639 | 0.238 | 3.17 (1 to 6) |
| Typical antipsychotic | 0.496 | 0.014 | 4.02 (2 to 6) |
| Placebo | 0.457 | 0.004 | 4.26 (2 to 6) |
| Statin | 0.344 | 0.030 | 4.93 (1 to 6) |
| CHE inhibitor | 0.002 | 0.000 | 6.99 (7 to 7) |
aMean rank with 2.5% and 97.5% quantiles in parentheses.
CHE: cholinesterase.
ICU: intensive care unit.
Pr: probability.
RE: random‐effects.
SUCRA: surface under the cumulative ranking curve.
5. b) Length of hospital stay
Pairwise meta‐analysis (direct comparisons)
Data from a total of nine trials (n = 1403 participants) contributed to the analysis of length of hospital stay (Atalan 2013; Devlin 2010; Girard 2018; Hakim 2012; Needham 2016; Page 2013; Page 2017; Reade 2016; van Eijk 2010); eight studies were placebo‐controlled. No pairwise comparison was statistically significant (Analysis 6.1;Figure 13).
6.1. Analysis.
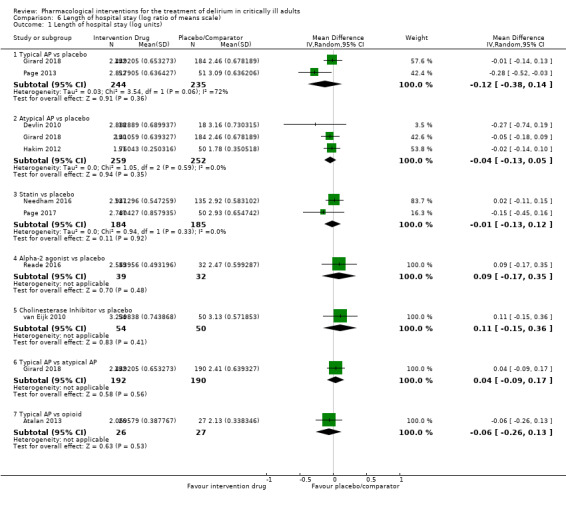
Comparison 6 Length of hospital stay (log ratio of means scale), Outcome 1 Length of hospital stay (log units).
13.
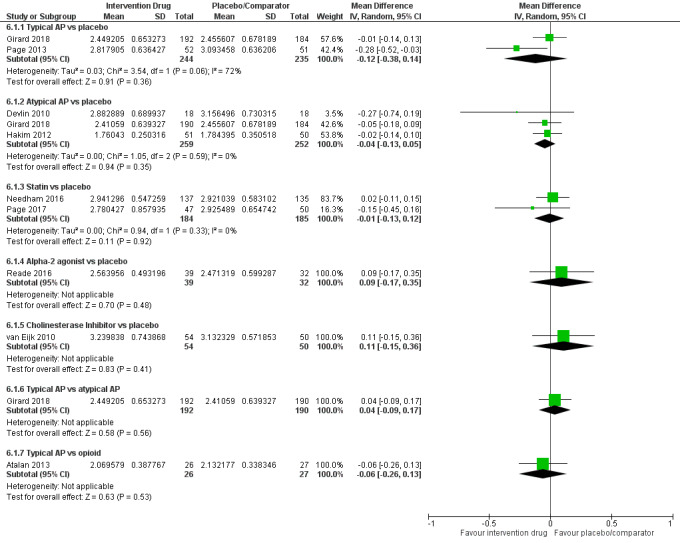
Forest plot of comparison: 6 Length of hospital stay (log units), outcome: 6.1 Length of hospital stay (log units).
NMA (combination of direct and indirect comparisons)
The forest plot in Figure 11 presents RoM estimates for each intervention compared to placebo from the random‐effects consistency model. The random‐effects consistency model was an adequate fit, with a posterior total residual deviance of 19.07 (compared to 19 unconstrained data points). The intervention with the smallest RoM versus placebo was typical antipsychotics (RoM 0.92, 95% CrI 0.65 to 1.18; SUCRA 0.722; low‐quality evidence). In order of descending SUCRA values, the next best interventions were atypical antipsychotics (RoM vs placebo 0.93, 95% CrI 0.69 to 1.16; SUCRA 0.693; moderate‐quality evidence), statins (RoM vs placebo 0.98, 95% CrI 0.69 to 1.30; SUCRA 0.537; moderate‐quality evidence), opioids (RoM vs placebo 0.97, 95% CrI 0.55 to 1.60; SUCRA 0.532; very low‐quality evidence), placebo (SUCRA 0.435), dexmedetomidine (RoM vs placebo 1.10, 95% CrI 0.69 to 1.75; SUCRA 0.301; moderate‐quality evidence), and rivastigmine (RoM vs placebo 1.11, 95% CrI 0.70 to 1.77; SUCRA 0.280; moderate‐quality evidence). Comparisons between active interventions are provided in Table 17, and secondary measures of effect are presented in Table 18. In all cases, 95% CrIs were wide and failed to rule out the possibility of no difference. The between‐study SD, as a measure of heterogeneity, was estimated to be 0.15 (95% CrI 0.005 to 0.53). Comparison of DIC values between the random‐effects consistency model (‐27.19), and the corresponding random‐effects unrelated means model (‐27.32), as well as inspection of a scatterplot of posterior mean deviance contributions from both models (Figure 7), did not suggest violation of the consistency assumption.
11. Length of hospital stay: league table of posterior median pairwise RoM and 95% CrI (lower triangle), and pairwise probabilities that a treatment is better than another (upper triangle).
| Typical antipsychotic | 0.546 | 0.673 | 0.654 | 0.810 | 0.828 | 0.820 |
| 0.99 (0.70, 1.38) |
Atypical antipsychotic |
0.651 | 0.604 | 0.801 | 0.818 | 0.827 |
| 0.94 (0.60, 1.43) | 0.95 (0.63, 1.42) | Statin | 0.498 | 0.716 | 0.736 | 0.596 |
| 0.94 (0.60, 1.47) | 0.95 (0.55, 1.67) | 1.00 (0.55, 1.88) | Opioid | 0.685 | 0.700 | 0.561 |
| 0.83 (0.46, 1.39) | 0.84 (0.48, 1.38) | 0.88 (0.49, 1.52) | 0.88 (0.42, 1.73) | Alpha2 agonist | 0.515 | 0.305 |
| 0.82 (0.46, 1.37) | 0.83 (0.47, 1.36) | 0.88 (0.49, 1.49) | 0.87 (0.41, 1.70) | 0.99 (0.51, 1.90) | CHE inhibitor | 0.278 |
| 0.92 (0.65, 1.18) | 0.93 (0.69, 1.16) | 0.98 (0.69, 1.30) | 0.97 (0.55, 1.60) | 1.10 (0.69, 1.75) | 1.11 (0.70, 1.77) | Placebo |
CHE: cholinesterase.
Crl: credible interval.
RoM: ratio of means.
Treatments other than placebo are in the order of decreasing surface under the cumulative ranking curve (SUCRA) value from upper left to lower right. For each comparison, the lower/right‐most treatment is the reference treatment. For example, the RoM estimate of 0.92 (95% CrI 0.65 to 1.18) in the lower triangle suggests an 8% reduction in mean length of hospital stay with typical antipsychotics compared to placebo. The corresponding probability estimate in the upper triangle suggests a probability of 82% that typical antipsychotics are better than placebo for length of hospital stay.
12. Length of hospital stay: mean SUCRA value, mean probability to be the best, and mean rank for each treatment.
| RE consistency model | |||
| Mean SUCRA | Mean Pr(best) | Mean ranka | |
| Typical antipsychotic | 0.722 | 0.235 | 2.67 (1 to 6) |
| Atypical antipsychotic | 0.693 | 0.218 | 2.84 (1 to 6) |
| Statin | 0.537 | 0.147 | 3.78 (1 to 7) |
| Opioid | 0.532 | 0.225 | 3.81 (1 to 7) |
| Placebo | 0.435 | 0.008 | 4.39 (2 to 6) |
| Alpha2 agonists | 0.301 | 0.090 | 5.19 (1 to 7) |
| CHE inhibitor | 0.280 | 0.078 | 5.32 (1 to 7) |
aMean rank with 2.5% and 97.5% quantiles in parentheses.
CHE: cholinesterase.
Pr: probability.
RE: random‐effects.
SUCRA: surface under the cumulative ranking curve.
6. Mortality
Pairwise meta‐analysis (direct comparisons)
Data from a total of 10 trials (n = 1584 participants) contributed to the analysis of mortality (Al‐Qadheeb 2016; Atalan 2013; Devlin 2010; Girard 2010a; Girard 2018; Hakim 2012; Needham 2016; Page 2013; Page 2017; van Eijk 2010). Studies were placebo‐controlled with cholinesterase inhibitors, typical and atypical antipsychotics, and statins as the interventions assessed, except for one trial (Atalan 2013), which compared opioids with typical antipsychotics. Mortality was reported at various time points and settings (e.g. 14 day, 28 day, ICU, hospital). No comparisons were statistically significant (Analysis 7.1;Figure 14).
7.1. Analysis.
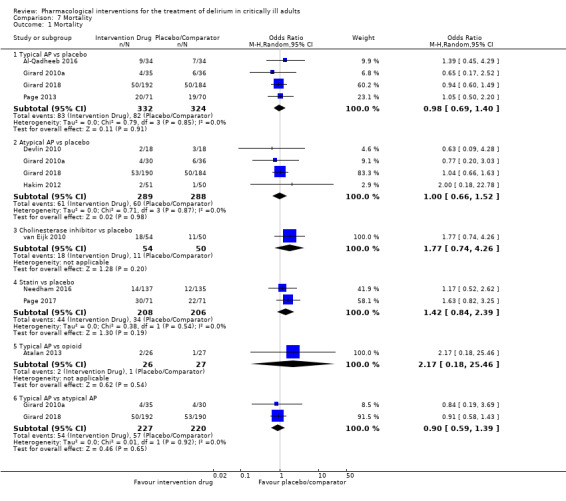
Comparison 7 Mortality, Outcome 1 Mortality.
14.
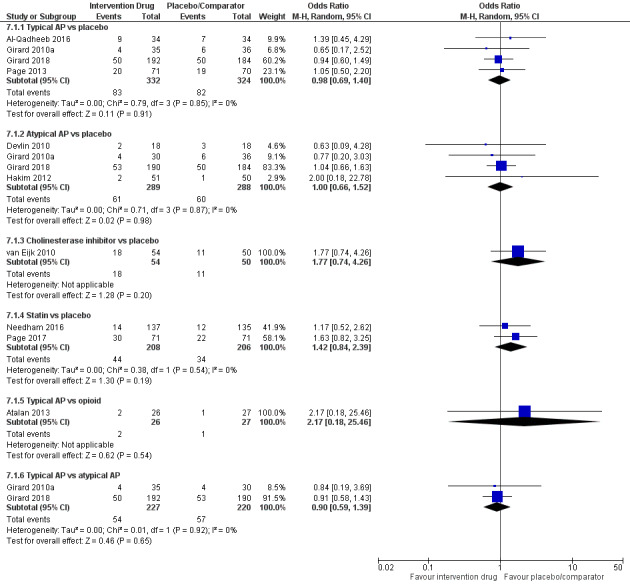
Forest plot of comparison: 7 Mortality, outcome: 7.1 Mortality.
NMA (combinations of direct and indirect comparisons)
We planned to perform an NMA for mortality. However, due to variability in follow‐up duration and settings across comparisons in the network, NMA was judged by the research team to be inappropriate and thus was not pursued. The disconnected network of interventions for the setting of ICU mortality alone (or hospital mortality alone) made NMA infeasible.
7. Use of physical restraint
Two studies reported on physical restraint application but used different outcome measures that were not amenable to meta‐analysis. Reade 2009 reported that 8/10 participants in the antipsychotic group were restrained compared to 9/10 participants in the alpha2 agonist group (no statistical difference), and van Eijk 2010 reported the percentage of days on which participants were restrained (no difference between groups was observed: 1% placebo and 1% cholinesterase inhibitor, respectively).
8. Hospital discharge disposition
Three studies reported on patient discharge disposition with insufficient information for pooling (Al‐Qadheeb 2016; Devlin 2010; Reade 2016). Devlin 2010 reported the combined outcome of home or rehabilitation facility (89% quetiapine vs 56% placebo; P = 0.06). Al‐Qadheeb 2016 reported no overall statistical difference in the percentage of participants discharged home (41.2% haloperidol vs 26.5% placebo), to a rehabilitation facility (29.4% haloperidol vs 47.1% placebo), or to long‐term care (2.9% haloperidol vs 2.9% placebo). Finally, Reade 2016 reported the percentage of participants transferred to rehabilitation facilities (13.2% dexmedetomidine vs 9.7% placebo; P = 0.65).
9. Long‐term cognitive outcome
This outcome was reported for only one trial (Page 2017). Study investigators assessed cognitive outcomes at six months using the Brief Test of Adult Cognition by Telephone (BTACT) (Lachman 2008). The BTACT assesses multiple dimensions central to effective cognitive functioning (e.g. episodic memory, reasoning, executive function). They also compared the Informant Questionnaire on Cognitive Decline in the Elderly (IQCODE) at baseline versus data at six‐month follow‐up (Jorm 1994). BTACT composite scores and differences between the IQCODE at baseline and at six‐month follow‐up did not differ between the two groups.
10. Health‐related quality of life
No study reported this outcome.
11. Adverse events ‐ a) Akathisia
Pairwise meta‐analysis (direct comparisons)
Akathisia was reported in two trials comparing antipsychotics to placebo (Girard 2010a; Page 2013). The overall number of participants was low (N = 242), as was the number of events. Akathisia was assessed subjectively with a 10‐cm visual analogue scale (Girard 2010a), or it was not specified how assessment was performed (Page 2013). We found no differences in any of the drug pairwise comparisons (Analysis 8.1; Figure 15). We assessed the evidence as low quality.
8.1. Analysis.
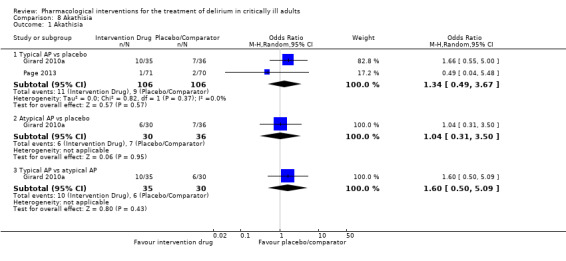
Comparison 8 Akathisia, Outcome 1 Akathisia.
15.
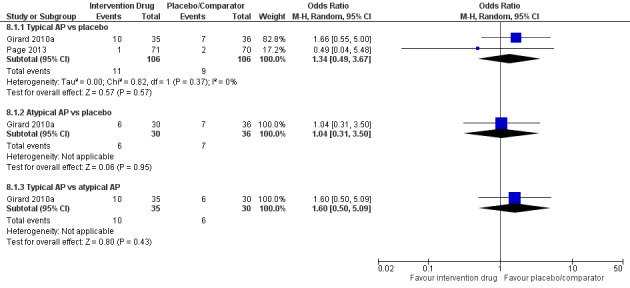
Forest plot of comparison: 8 Akathisia, outcome: 8.1 Akathisia.
NMA (combination of direct and indirect comparisons)
We did not conduct an NMA for this adverse event as only trials investigating antipsychotic drugs reported on this outcome.
11. Adverse events ‐ b) Arrhythmia and QTc prolongation
Pairwise meta‐analysis (direct comparisons)
Arrhythmias were reported as an adverse event in four trials (Girard 2010a; Girard 2018; Page 2013; Reade 2009), and 828 participants were analysed. These trials compared antipsychotics ‐ in Girard 2010a,Girard 2018, and Page 2013 ‐ versus placebo or dexmedetomidine ‐ in Reade 2009. The number of included participants was small, and events were rare. Only typical antipsychotics compared with placebo were associated with significantly increased odds of arrhythmias (OR 3.09, 95% CI 1.11 to 8.62) amongst all pairwise comparisons (Analysis 9.1; Figure 16).
9.1. Analysis.
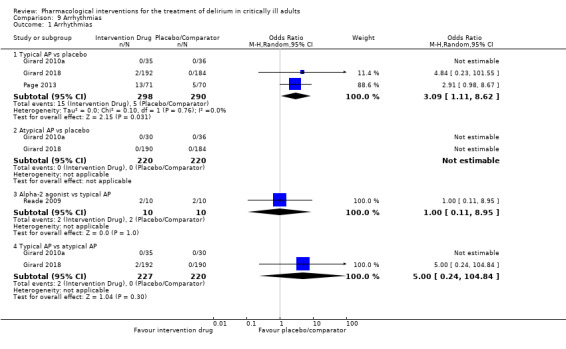
Comparison 9 Arrhythmias, Outcome 1 Arrhythmias.
16.
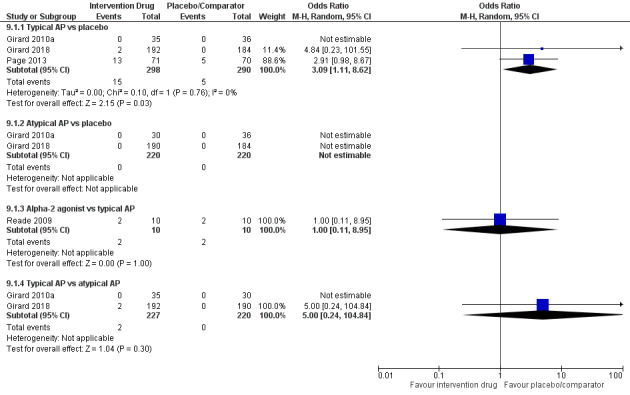
Forest plot of comparison: 9 Arrhythmias, outcome: 9.1 Arrhythmias.
QTc prolongation, measured by electrocardiogram, was reported in seven studies (Al‐Qadheeb 2016; Bakri 2015; Devlin 2010; Girard 2010a; Girard 2018; Page 2013; Reade 2009), and 996 participants were analysed. Trials investigated antipsychotics compared to placebo, ondansetron, and dexmedetomidine. The overall number of participants was small, as was the number or reported events. No comparisons were statistically significant (Analysis 10.1; Figure 17).
10.1. Analysis.
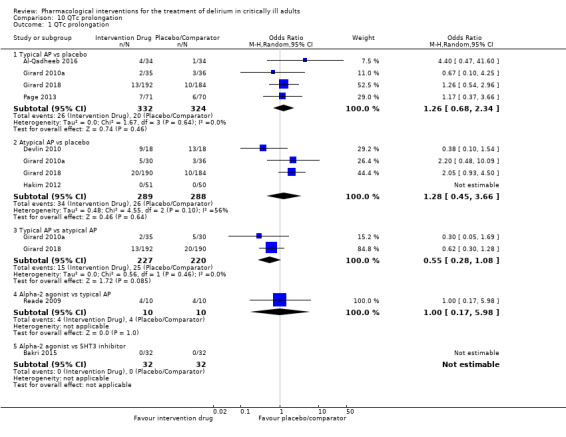
Comparison 10 QTc prolongation, Outcome 1 QTc prolongation.
17.
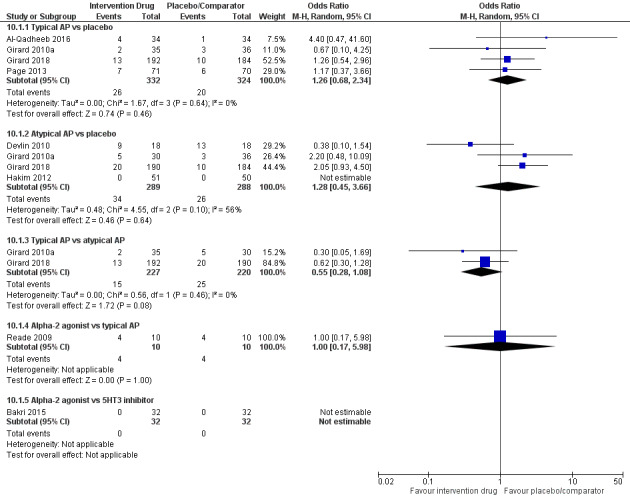
Forest plot of comparison: 10 QTc prolongation, outcome: 10.1 QTc prolongation.
NMA (combination of direct and indirect comparisons)
We did not conduct an NMA for this adverse event.
11. Adverse events ‐ c) Extrapyramidal side effects
Pairwise meta‐analysis (direct comparisons)
Extrapyramidal side effects were assessed in six antipsychotic trials (Al‐Qadheeb 2016; Devlin 2010; Girard 2010a; Girard 2018; Page 2013; Skrobik 2004), which included a total of 985 analysed participants. Extrapyramidal symptoms were assessed on the modified Simpson‐Angus Scale in five trials (Devlin 2010; Girard 2010a; Girard 2018; Page 2013; Skrobik 2004), and one trial did not report the assessment method used (Al‐Qadheeb 2016). Pooled results showed no significant differences compared to placebo (Analysis 11.1;Figure 18).
11.1. Analysis.
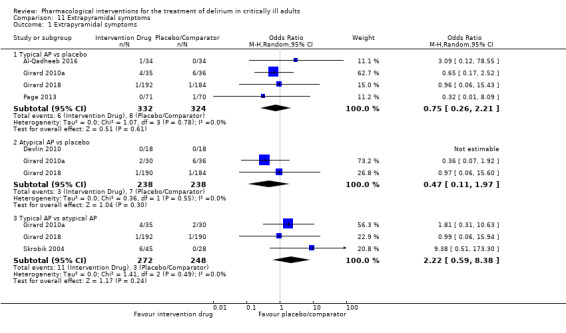
Comparison 11 Extrapyramidal symptoms, Outcome 1 Extrapyramidal symptoms.
18.
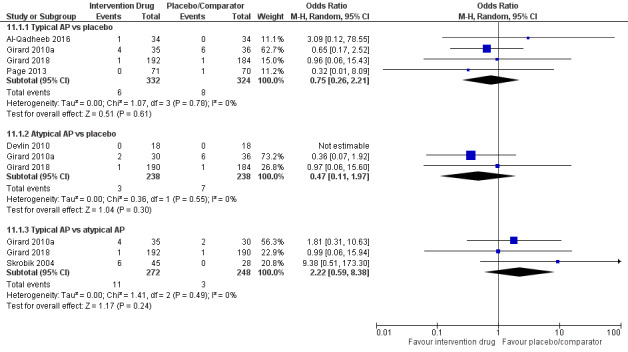
Forest plot of comparison: 11 Extrapyramidal symptoms, outcome: 11.1 Extrapyramidal symptoms.
NMA (combinations of direct and indirect comparisons)
We planned to perform an NMA for extrapyramidal side effects. However, due to both the rare nature of events in this analysis and violation of the consistency assumption, NMA was judged to be inappropriate.
11. Adverse events ‐ d) Seizures
No trial reported or examined seizures as an outcome.
Subgroup analyses
We planned to explore subgroup analyses or meta‐regression analyses, or both, to address the impact of age, ICU patient population, delirium subtype, and use of non‐drug co‐interventions on our findings to establish their robustness.
Neither subgroup analyses nor meta‐regression analyses were feasible to explore the delirium subtype (e.g. hyperactive, hypoactive, mixed) and use of non‐drug co‐interventions. We did not have a well‐connected evidence network to perform subgroup analyses for studies with mean participant age ≥ 65 years. Subgroup analyses for studies with mean age < 65 years resulted in widened CIs/CrIs for typical and atypical antipsychotics and disappearance of opioids and cholinesterase inhibitors from the evidence network, but did not provide different results compared to overall analyses.
Sensitivity analyses
We explored some sensitivity analyses involving alternative geometries of the network. We did not end up with a well‐connected evidence network for each outcome once high risk of bias trials were excluded. There were insufficient trials to conduct analyses involving alternative geometries based on dose or frequency of drug administration. Planned sensitivity analyses collapsing atypical and typical antipsychotics into one node did not provide different results compared to the overall analyses.
After exclusion of studies that focused on subsyndromal delirium (Al‐Qadheeb 2016; Hakim 2012), CIs/CrIs widened for typical and atypical antipsychotics, but results were not different compared to findings of the overall analyses.
Removal from analysis of studies with patients of low illness severity eliminated alpha2 agonists (dexmedetomidine) and opioids (morphine) from the evidence network (Atalan 2013;Hakim 2012;Reade 2016), but results for the remaining interventions were not different compared to findings of the overall analyses.
Reporting bias
We did not produce a funnel plot for each pairwise comparison. To detect small‐study effects by checking asymmetry per pairwise comparison is not feasible due to the low number of identified trials.
Discussion
Summary of main results
We identified 14 randomized controlled trials (RCTs) that enrolled 1844 adult participants which evaluated pharmacological treatments for delirium in the intensive care unit (ICU). These trials evaluated six different drug classes, primarily comparing one active drug versus placebo. Most trials were small, enrolling fewer than 100 participants. Nine trials scored low risk of bias across all domains; the overall quality of evidence for each outcome was assessed via the GRADE approach; quality ranged from low to high.
Pairwise meta‐analyses showed that only the alpha2 agonist dexmedetomidine (vs placebo) significantly reduced the duration of delirium in critically ill adults with delirium; this was based on a single study with < 100 participants. Network meta‐analysis shows that the smallest ratio of means (vs placebo) was associated with the alpha2 agonist dexmedetomidine, followed by atypical antipsychotics. However, effect sizes for either of the drug classes were neither statistically nor clinically significant. Among secondary outcomes, network meta‐analysis (NMA) revealed that only dexmedetomidine was associated with a shorter duration of mechanical ventilation, and that the cholinesterase (CHE) inhibitor rivastigmine was associated with longer ICU stay. Otherwise, no pharmacological intervention was found to achieve statistical or clinical significance for the secondary outcomes. Analyses of reported adverse drug events found that events were similar to those seen with placebo. The 10 ongoing studies and the six studies awaiting classification that we identified, once published and assessed, may alter the conclusions of this review.
Please notice that the 95% credible intervals from Bayesian NMA results are generally more conservative (wider) than the corresponding 95% confidence intervals from pairwise meta‐analyses. If a pairwise comparison had at least one study contributing direct evidence to NMA and resulted in a 95% credible interval that ruled out the possibility of no difference, the corresponding 95% confidence interval from pairwise meta‐analysis was also significant.
Overall completeness and applicability of evidence
When we designed the protocol (Burry 2015), for several reasons we anticipated at least 20 trials specifically investigating various pharmacological interventions for the treatment of ICU delirium. These reasons included the inclusion of delirium as a quality indicator in care of the elderly, poor outcomes associated with delirium in critically ill patients, and the number of registered trials, as well as the strong recommendations for delirium prevention and treatment provided in the Society of Critical Care Medicine's pain, agitation, and delirium guidelines (Barr 2013). Using strong literature review methods, we identified only 14 published trials that matched our review questions. However, we did identify six studies awaiting classification and 10 ongoing trials, several of which are large‐scale, multi‐centre trials. This suggests that this topic will expand greatly in the next five years. We found that most trials, and those ongoing, examined use of pharmacological interventions commonly given in clinical practice, primarily antipsychotics and the alpha2 agonist dexmedetomidine. We found sufficient data to conduct pairwise comparisons and NMA to answer our primary outcome of interest, but we could not analyse some of the secondary outcomes that we deemed clinically important, as these outcomes were not investigated in any trial (i.e. relapse, resolution of symptoms, long‐term cognitive outcomes, and health‐related quality of life). Nor did we find sufficient information to conduct our planned subgroup analyses on age, ICU population type, delirium subtype, or use of non‐pharmacological co‐interventions.
Quality of the evidence
We scored the risk of bias for each trial and used GRADEpro software to inform the generation of evidence quality statements. Among the 14 RCTs included in this review, nine trials scored low risk of bias across all domains. We judged available evidence to range from low to high quality. Evidence for the primary outcome ‐ duration of delirium ‐ was of moderate to high quality when each drug class was compared to placebo. We most commonly downgraded this evidence for imprecision.
Potential biases in the review process
This review followed Cochrane's systematic review procedures closely, with only minor amendments to the published protocol (Burry 2015). Our search was exhaustive without restrictions; therefore we believe we have evaluated the available evidence in full. The trials included in our review directly examined our chosen population and the primary outcome ‐ duration of delirium ‐ as their primary or secondary outcome. We had originally set the primary outcome to be duration of delirium, defined as time from which it wasfirst identified to when it was first resolved (i.e. screened negative as defined by study authors (e.g. first negative screen, two consecutive screenings)), and our secondary outcome to be duration of delirium (as defined by study authors). We found far more variability in the definition of the outcome used than we had anticipated; thus we ended up reporting only the duration of delirium for pooling of results. The definition applied by study authors also varied, with some using 24 hours without delirium, some 48 hours, and others not reporting the definition they applied.
For continuous outcomes, we approximated means and standard deviations (SDs) from medians and interquartile ranges (IQRs) (Wan 2014) to make use of studies that reported only medians and IQRs for some outcomes. Before all ratio of means (RoM) analyses, we transformed means and SDs to the log scale (Higgins 2008) to overcome various time windows across studies with existing pairwise meta‐analysis and NMA methods, and to make evidence synthesis possible. The first transformation may not always yield accurate RoM estimates for skewed outcomes in small studies. Despite the robust properties of the second transformation for skewed outcomes (Higgins 2008), interpretation of RoM analyses is challenging. We did not approximate means and SDs using any range‐related formulae.
We are not aware of other potential sources of bias.
Agreements and disagreements with other studies or reviews
This is the first NMA examining treatment of delirium for ICU patients, and it is the first Cochrane systematic review examining pharmacological interventions for ICU delirium. We identified two recent systematic reviews examining antipsychotics for prevention or treatment of delirium, or both, in any hospital population (i.e. ICU and non‐ICU) (Kishi 2016; Neufeld 2016). Our findings regarding antipsychotics are consistent with those of Neufeld 2016, in that antipsychotics had no effect on delirium duration when review authors pooled the results of treatment trials. Kishi 2016 conducted a review examining antipsychotics for prevention or treatment of delirium, or both, in any hospital population, including data from four studies that were unpublished or were published in abstract form only. The review by Kishi reported response rate (response rate at the study endpoint examining many different severity and global scales) and did not report on duration of delirium. Pooled results for response rate showed that antipsychotics were superior to placebo and non‐antipsychotic drugs. We identified one Cochrane systematic review on alpha2 agonists for long‐term sedation during mechanical ventilation in critically ill patients, which examined risk of delirium as a secondary outcome (Chen 2015); review authors did not report on duration of delirium nor on other delirium outcomes that we reported. The Chen review found no evidence that dexmedetomidine decreased the risk of delirium (risk ratio 0.85, 95% CI 0.63 to 1.14; seven studies; 1624 participants; low‐quality evidence) compared to traditional sedatives.
Authors' conclusions
Implications for practice.
In clinical practice, pharmacological interventions are commonly administered to critically ill patients to manage their symptoms of delirium (Burry 2017). We found evidence that the alpha2 agonist dexmedetomidine may have some role in shortening delirium duration, although this small effect was seen in pairwise analyses based on a single small study compared with placebo, and was not seen in the NMA results. No other pharmacological intervention including antipsychotics, the most commonly prescribed drug for delirium treatment, had any effect on delirium duration nor on any of our a priori selected secondary outcomes. It is also important to note that the cholinesterase inhibitor rivastigmine was associated with harm, and as such, guidelines suggest against its use for treatment of ICU delirium. The 10 ongoing studies and the six studies awaiting classification, once published and assessed, may alter the conclusions of this review; therefore, their results are much anticipated. The frequency of prescribing these drug classes for critically ill adults with delirium and the non‐significant findings of our review should be considered at the bedside and should be incorporated into future pain, agitation, and delirium guidelines.
Implications for research.
We identified 10 ongoing studies, of which seven have a large target enrolment number (100 to 1000 participants), suggesting growing interest in the treatment of ICU delirium. These RCTs should strengthen our results and may potentially alter the direction of our findings. For example, five ongoing trials are examining antipsychotics and three are examining the alpha2 agonist dexmedetomidine ‐ the drug classes found most promising in our analysis ‐ each trial with large target enrolment.
We note the promise of many new treatment trials on the horizon; however, we must acknowledge the need to standardize outcome reporting in ICU delirium trials to permit maximum pooling and interpretation of results. We found far greater variability in the definitions of study outcomes used than we had anticipated, which led us to modify our primary outcome and to limit pooling for some outcomes (e.g. mortality). We found no reporting on some clinically important outcomes such as symptom management (e.g. treating agitation, stopping treatment interferences) and long‐term cognitive outcomes, and we found new outcomes not listed in our protocol (e.g. number of days in coma) in multiple new RCTs and ongoing trials. The Del‐COrS ("Developmnt of core outcome sets for effectiveness trial of interventions to prevent and/or treat delirium") Group is leading the development of international consensus on outcomes for trials of intervention to prevent and treat delirium in multiple patient populations (Rose 2017). Findings from this group should be used to guide future ICU delirium trials.
We also found that RCTs in this review rarely reported on the use of non‐pharmacological strategies. Among the trials that we identified, all but one showed poor utilization of non‐pharmacological strategies. For example, early mobilization has been shown to reduce the duration of delirium (Barr 2013), and its use in practice is encouraged. Therefore, future trials should clearly describe the use of such strategies in their methods and should report compliance in their results. We also found poor reporting on the use of physical restraints ‐ a non‐pharmacological intervention associated with delirium and prolonged duration of delirium (Rose 2016).
What's new
| Date | Event | Description |
|---|---|---|
| 4 September 2019 | Amended | Contact details updated. |
History
Protocol first published: Issue 6, 2015 Review first published: Issue 9, 2019
| Date | Event | Description |
|---|---|---|
| 3 January 2019 | Amended | Editorial team changed to Cochrane Emergency and Critical Care |
Acknowledgements
We would like to express our gratitude to Melanie Guenette, Research Co‐ordinator, who supported the protocol and review process. We would like to acknowledge Becky Skidmore and Kaitryn Campbell for assistance in generating and testing our search strategies. We would also like to thank Anna Lee (Content Editor); Nathan Pace (Statistical Editor); Russel J Roberts, John W Devlin, and Matthew S Duprey (Peer Reviewers); Janet Wale (Consumer Editor); Jane Cracknell and Teo Quay (Managing Editors); and Harald Herkner (Co‐ordinating Editor) for help and editorial advice provided during preparation of this systematic review.
Appendices
Appendix 1. RCT Search Strategy: Ovid MEDLINE®, Ovid MEDLINE® In‐Process & Other Non‐ Indexed Citations, Embase Classic+Embase, and PsycINFO
Search Strategy:
‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐
1 ((postoperati* or post‐operati* or postsurg* or post‐surg*) adj1 ("cognitive dysfunction" or "brain dysfunction")).tw.
2 Intensive Care Units/
3 Burn Units/
4 Coronary Care Units/
5 Respiratory Care Units/
6 exp Intensive Care Units, Pediatric/
7 exp Critical Care/
8 ((intensive or critical or acute) adj3 care).tw.
9 (ICU or ICUs or NICU or NICUs or PICU or PICUs or SICU or SICUs or CCU or CCUs).tw.
10 (burn$1 adj3 (unit$1 or centre$1 or center$1)).tw.
11 ((cardiac or coronary or heart) adj3 (unit$1 or centre$1 or center$1)).tw.
12 (respiratory adj3 (unit$1 or centre$1 or center$1)).tw.
13 ((surgical or surger*) adj3 (unit$1 or centre$1 or center$1)).tw.
14 Postoperative Care/
15 Postoperative Complications/
16 (postoperati* or post‐operati* or postsurg* or post‐surg*).tw.
17 Critical Illness/
18 (critical* adj (ill or illness*)).tw.
19 or/2‐18
20 Delirium/
21 deliri*.tw.
22 Psychoses, Substance‐Induced/
23 (psychos* adj3 (toxic* or exogenous* or chemical* or drug or drugs or medication* or substance*)).tw.
24 (acute brain adj (dysfunction* or failure* or syndrome*)).tw.
25 (cloud* adj3 consciousness*).tw.
26 clouded state*.tw.
27 ((psycho‐organic syndrome* or psychoorganic syndrome* or organic psychosyndrome* or organic psycho‐syndrome*) adj3 acute).tw.
28 exp Confusion/ci
29 Hallucinations/
30 hallucinat*.tw.
31 or/20‐30
32 19 and 31
33 1 or 32
34 (controlled clinical trial or randomized controlled trial).pt.
35 clinical trials as topic.sh.
36 (randomi#ed or randomly or RCT$1 or placebo*).tw.
37 ((singl* or doubl* or trebl* or tripl*) adj (mask* or blind* or dumm*)).tw.
38 trial.ti.
39 or/34‐38
40 33 and 39
41 exp Animals/ not (exp Animals/ and Humans/)
42 40 not 41
43 (comment or editorial or interview or letter or news).pt.
44 42 not 43
45 44 use prmz
46 remove duplicates from 45 [MEDLINE RECORDS]
47 postoperative delirium/
48 postoperative cognitive dysfunction/
49 ((postoperati* or post‐operati* or postsurg* or post‐surg*) adj1 ("cognitive dysfunction" or "brain dysfunction")).tw.
50 intensive care psychosis/
51 or/47‐50
52 intensive care unit/
53 burn unit/
54 coronary care unit/
55 intensive care/
56 ((intensive or critical or acute) adj3 care).tw.
57 (ICU or ICUs or NICU or NICUs or PICU or PICUs or SICU or SICUs or CCU or CCUs).tw.
58 (burn$1 adj3 (unit$1 or centre$1 or center$1)).tw.
59 ((cardiac or coronary or heart) adj3 (unit$1 or centre$1 or center$1)).tw.
60 (respiratory adj3 (unit$1 or centre$1 or center$1)).tw.
61 ((surgical or surger*) adj3 (unit$1 or centre$1 or center$1)).tw.
62 postoperative care/
63 postoperative complication/
64 (postoperati* or post‐operati* or postsurg* or post‐surg*).tw.
65 critical illness/
66 (critical* adj (ill or illness*)).tw.
67 or/52‐66
68 exp delirium/
69 deliri*.tw.
70 (psychos* adj3 (toxic* or exogenous* or chemical* or drug or drugs or medication* or substance*)).tw.
71 (acute brain adj (dysfunction* or failure* or syndrome*)).tw.
72 (cloud* adj3 consciousness*).tw.
73 clouded state*.tw.
74 ((psycho‐organic syndrome* or psychoorganic syndrome* or organic psychosyndrome* or organic psycho‐syndrome*) adj3 acute).tw.
75 exp hallucination/
76 hallucinat*.tw.
77 or/68‐76
78 67 and 77
79 51 or 78
80 randomized controlled trial/ or controlled clinical trial/
81 exp "clinical trial (topic)"/
82 (randomi#ed or randomly or RCT$1 or placebo*).tw.
83 ((singl* or doubl* or trebl* or tripl*) adj (mask* or blind* or dumm*)).tw.
84 trial.ti.
85 or/80‐84
86 79 and 85
87 exp animal experimentation/ or exp models animal/ or exp animal experiment/ or nonhuman/ or exp vertebrate/
88 exp humans/ or exp human experimentation/ or exp human experiment/
89 87 not 88
90 86 not 89
91 (editorial or letter).pt.
92 90 not 91
93 92 use emczd
94 remove duplicates from 93 [EMBASE RECORDS]
95 ((postoperati* or post‐operati* or postsurg* or post surg*) adj1 ("cognitive dysfunction" or "brain dysfunction")).tw.
96 exp intensive care/
97 ((intensive or critical or acute) adj3 care).tw.
98 (ICU or ICUs or NICU or NICUs or PICU or PICUs or SICU or SICUs or CCU or CCUs).tw.
99 (burn$1 adj3 (unit$1 or centre$1 or center$1)).tw.
100 ((cardiac or coronary or heart) adj3 (unit$1 or centre$1 or center$1)).tw.
101 (respiratory adj3 (unit$1 or centre$1 or center$1)).tw.
102 ((surgical or surger*) adj3 (unit$1 or centre$1 or center$1)).tw.
103 postsurgical complications/
104 (postoperati* or post‐operati* or postsurg* or post‐surg*).tw.
105 (critical* adj (ill or illness*)).tw.
106 or/96‐105
107 delirium/
108 deliri*.tw.
109 (psychos* adj3 (toxic* or exogenous* or chemical* or drug or drugs or medication* or substance*)).tw.
110 (acute brain adj (dysfunction* or failure* or syndrome*)).tw.
111 (cloud* adj3 consciousness*).tw.
112 clouded state*.tw.
113 ((psycho‐organic syndrome* or psychoorganic syndrome* or organic psychosyndrome* or organic psycho‐syndrome*) adj3 acute).tw.
114 mental confusion/
115 exp Hallucinations/
116 hallucinat*.tw.
117 or/107‐116
118 106 and 117
119 95 or 118
120 clinical trials/
121 (randomi#ed or randomly or RCT$1 or placebo*).tw.
122 ((singl* or doubl* or trebl* or tripl*) adj (mask* or blind* or dumm*)).tw.
123 trial.ti.
124 or/120‐123
125 119 and 124
126 exp Animals/ not (exp Animals/ and Humans/)
127 125 not 126
128 127 use prmz
129 127 use emczd
130 127 not (128 or 129) [PSYCINFO RECORDS]
131 remove duplicates from 130
132 46 or 94 or 131
133 remove duplicates from 132 [TOTAL UNIQUE HITS]
134 133 use prmz [MEDLINE UNIQUE HITS]
135 133 use emczd [EMBASE UNIQUE HITS]
136 133 not (134 or 135) [PSYCINFO UNIQUE HITS]
Appendix 2. RCT Search Strategy: CCTR, DSR, DARE, CENTRAL, HTA, and NHS SEED
ID Search Hits
#1 (postoperati* or (post next operati*) or postsurg* or (post next surg*)) next ("cognitive dysfunction" or "brain dysfunction"):ti,ab,kw
#2 [mh ^"Intensive Care Units"]
#3 [mh "Burn Units"]
#4 [mh "Coronary Care Units"]
#5 [mh "Respiratory Care Units"]
#6 [mh "Intensive Care Units, Pediatric"]
#7 [mh "Critical Care"]
#8 ((intensive or critical or acute) near/4 care):ti,ab,kw
#9 (ICU or ICUs or NICU or NICUs or PICU or PICUs or SICU or SICUs or CCU or CCUs):ti,ab,kw
#10 (burn or burns) near/4 (unit or units or centre or centres or center or centers):ti,ab,kw
#11 (cardiac or coronary or heart) near/4 (unit or units or centre or centres or center or centers):ti,ab,kw
#12 respiratory near/4 (unit or units or centre or centres or center or centers):ti,ab,kw
#13 (surgical or surger*) near/4 (unit or units or centre or centres or center or centers):ti,ab,kw
#14 [mh "Postoperative Care"]
#15 [mh "Postoperative Complications"]
#16 postoperati* or (post next operati*) or postsurg* or (post next surg*):ti,ab,kw
#17 [mh "Critical Illness"]
#18 critical* next (ill or illness*):ti,ab,kw
#19 {or #2‐#18}
#20 [mh Delirium]
#21 deliri*:ti,ab,kw
#22 [mh ^"Psychoses, Substance‐Induced"]
#23 psychos* near/4 (toxic* or exogenous* or chemical* or drug or drugs or medication* or substance*):ti,ab,kw
#24 "acute brain" next (dysfunction* or failure* or syndrome*):ti,ab,kw
#25 cloud* near/4 consciousness*:ti,ab,kw
#26 clouded next state*:ti,ab,kw
#27 ("psycho‐organic" next syndrome*) or (psychoorganic next syndrome*) or (organic next psychosyndrome*) or (organic next psycho‐syndrome*) near/4 acute:ti,ab,kw
#28 [mh Confusion/ci]
#29 [mh Hallucinations]
#30 hallucinat*:ti,ab,kw
#31 {or #20‐#30}
#32 #19 and #31
#33 #1 or #32
DSR – (did not download – RCT search only)
DARE – (did not download – RCT search only)
CENTRAL ‐
HTA – (did not download – RCT search only)
NHS EED – (did not download – RCT search only)
Appendix 3. RCT Search Strategy: CINAHL
| # | Query | Limiters/Expanders |
| S42 | S33 AND S40 | Limiters ‐ Exclude MEDLINE records Expanders ‐ Apply related words Search modes ‐ Boolean/Phrase |
| S41 | S33 AND S40 | Expanders ‐ Apply related words Search modes ‐ Boolean/Phrase |
| S40 | s34 or s35 or s36 or s37 or s38 or s39 | Expanders ‐ Apply related words Search modes ‐ Boolean/Phrase |
| S39 | TI trial | Expanders ‐ Apply related words Search modes ‐ Boolean/Phrase |
| S38 | TI ( (singl* or doubl* or trebl* or tripl*) w1 (mask* or blind* or dumm*) ) OR AB ( (singl* or doubl* or trebl* or tripl*) w1 (mask* or blind* or dumm*) ) | Expanders ‐ Apply related words Search modes ‐ Boolean/Phrase |
| S37 | TI ( randomised or randomized or randomly or RCT or RCTs or placebo* ) OR AB ( randomised or randomized or randomly or RCT or RCTs or placebo* ) | Expanders ‐ Apply related words Search modes ‐ Boolean/Phrase |
| S36 | TI randomised or randomized or randomly or RCT or RCTs or placebo* | Expanders ‐ Apply related words Search modes ‐ Boolean/Phrase |
| S35 | (MH "Clinical Trials+") | Expanders ‐ Apply related words Search modes ‐ Boolean/Phrase |
| S34 | (MH "Randomized Controlled Trials") | Expanders ‐ Apply related words Search modes ‐ Boolean/Phrase |
| S33 | S1 OR S32 | Expanders ‐ Apply related words Search modes ‐ Boolean/Phrase |
| S32 | S19 AND S31 | Expanders ‐ Apply related words Search modes ‐ Boolean/Phrase |
| S31 | S20 OR S21 OR S22 OR S23 OR S24 OR S25 OR S26 OR S27 OR S28 OR S29 OR S30 | Expanders ‐ Apply related words Search modes ‐ Boolean/Phrase |
| S30 | TI hallucinat* OR AB hallucinat* | Expanders ‐ Apply related words Search modes ‐ Boolean/Phrase |
| S29 | (MH "Hallucinations+") | Expanders ‐ Apply related words Search modes ‐ Boolean/Phrase |
| S28 | (MH "Confusion+/CI") | Expanders ‐ Apply related words Search modes ‐ Boolean/Phrase |
| S27 | TI ( ("psycho‐organic" w1 syndrome*) or (psychoorganic w1 syndrome*) or (organic w1 psychosyndrome*) or "organic psycho‐syndrome" or "organic psycho‐syndromes") n3 acute ) OR AB ( ("psycho‐organic" w1 syndrome*) or (psychoorganic w1 syndrome*) or (organic w1 psychosyndrome*) or "organic psycho‐syndrome" or "organic psycho‐syndromes") n3 acute ) | Expanders ‐ Apply related words Search modes ‐ Boolean/Phrase |
| S26 | TI clouded w1 state* OR AB clouded w1 state* | Expanders ‐ Apply related words Search modes ‐ Boolean/Phrase |
| S25 | TI cloud* n3 consciousness* OR AB cloud* n3 consciousness* | Expanders ‐ Apply related words Search modes ‐ Boolean/Phrase |
| S24 | TI ( "acute brain" n1 (dysfunction* or failure* or syndrome*) ) OR AB ( "acute brain" n1 (dysfunction* or failure* or syndrome*) ) | Expanders ‐ Apply related words Search modes ‐ Boolean/Phrase |
| S23 | TI ( psychos* n3 (toxic* or exogenous* or chemical* or drug or drugs or medication* or substance*) ) OR AB ( psychos* n3 (toxic* or exogenous* or chemical* or drug or drugs or medication* or substance*) ) | Expanders ‐ Apply related words Search modes ‐ Boolean/Phrase |
| S22 | (MH "Psychoses, Substance‐Induced") | Expanders ‐ Apply related words Search modes ‐ Boolean/Phrase |
| S21 | TI deliri* OR AB deliri* | Expanders ‐ Apply related words Search modes ‐ Boolean/Phrase |
| S20 | (MH "Delirium") | Expanders ‐ Apply related words Search modes ‐ Boolean/Phrase |
| S19 | S2 OR S3 OR S4 OR S5 OR S6 OR S7 OR S8 OR S9 OR S10 OR S11 OR S12 OR S13 OR S14 OR S15 OR S16 OR S17 OR S18 | Expanders ‐ Apply related words Search modes ‐ Boolean/Phrase |
| S18 | TI ( critical* w1 (ill or illness*) ) OR AB ( critical* w1 (ill or illness*) ) | Expanders ‐ Apply related words Search modes ‐ Boolean/Phrase |
| S17 | (MH "Critical Illness") | Expanders ‐ Apply related words Search modes ‐ Boolean/Phrase |
| S16 | TI ( postoperati* or post‐operati* or postsurg* or post‐surg* ) OR AB ( postoperati* or post‐operati* or postsurg* or post‐surg* ) | Expanders ‐ Apply related words Search modes ‐ Boolean/Phrase |
| S15 | (MH "Postoperative Complications+") | Expanders ‐ Apply related words Search modes ‐ Boolean/Phrase |
| S14 | (MH "Postoperative Care+") | Expanders ‐ Apply related words Search modes ‐ Boolean/Phrase |
| S13 | TI ( ((surgical or surger*) n3 (unit or units or centre or centres or center or centers)) ) OR AB ( ((surgical or surger*) n3 (unit or units or centre or centres or center or centers)) ) | Expanders ‐ Apply related words Search modes ‐ Boolean/Phrase |
| S12 | TI ( respiratory n3 (unit or units or centre or centres or center or centers) ) OR AB ( respiratory n3 (unit or units or centre or centres or center or centers) ) | Expanders ‐ Apply related words Search modes ‐ Boolean/Phrase |
| S11 | TI ( (cardiac or coronary or heart) n3 (unit or units or centre or centres or center or centers)) ) OR AB ( ((cardiac or coronary or heart) n3 (unit or units or centre or centres or center or centers)) ) | Expanders ‐ Apply related words Search modes ‐ Boolean/Phrase |
| S10 | TI ( ((burn or burns) n3 (unit or units or centre or centres or center or centers)) ) OR AB ( ((burn or burns) n3 (unit or units or centre or centres or center or centers)) ) | Expanders ‐ Apply related words Search modes ‐ Boolean/Phrase |
| S9 | TI ( (ICU or ICUs or NICU or NICUs or PICU or PICUs or SICU or SICUs or CCU or CCUs) ) OR AB ( (ICU or ICUs or NICU or NICUs or PICU or PICUs or SICU or SICUs or CCU or CCUs) ) | Expanders ‐ Apply related words Search modes ‐ Boolean/Phrase |
| S8 | TI ( ((intensive or critical or acute) n3 care) ) OR AB ( ((intensive or critical or acute) n3 care) ) | Expanders ‐ Apply related words Search modes ‐ Boolean/Phrase |
| S7 | (MH "Critical Care+") | Expanders ‐ Apply related words Search modes ‐ Boolean/Phrase |
| S6 | (MH "Respiratory Care Units") | Expanders ‐ Apply related words Search modes ‐ Boolean/Phrase |
| S5 | (MH "Coronary Care Units") | Expanders ‐ Apply related words Search modes ‐ Boolean/Phrase |
| S4 | (MH "Burn Units") | Expanders ‐ Apply related words Search modes ‐ Boolean/Phrase |
| S3 | (MH "Intensive Care Units, Neonatal") OR (MH "Intensive Care Units, Pediatric+") | Expanders ‐ Apply related words Search modes ‐ Boolean/Phrase |
| S2 | (MH "Intensive Care Units+") | Expanders ‐ Apply related words Search modes ‐ Boolean/Phrase |
| S1 | TI ( ((postoperati* or post‐operati* or postsurg* or post‐surg*) n1 ("cognitive dysfunction" or "brain dysfunction")) ) OR AB ( ((postoperati* or post‐operati* or postsurg* or post‐surg*) n1 ("cognitive dysfunction" or "brain dysfunction")) ) | Expanders ‐ Apply related words Search modes ‐ Boolean/Phrase |
Appendix 4. Web of Science
| # 41 | #39 NOT #40 Indexes=SCI‐EXPANDED, SSCI, A&HCI, CPCI‐S, CPCI‐SSH Timespan=All years |
| # 40 | (#39) ANDDOCUMENT TYPES: (Editorial Material OR Letter OR News Item) Indexes=SCI‐EXPANDED, SSCI, A&HCI, CPCI‐S, CPCI‐SSH Timespan=All years |
| # 39 | #38 AND #31 Indexes=SCI‐EXPANDED, SSCI, A&HCI, CPCI‐S, CPCI‐SSH Timespan=All years |
| # 38 | #37 OR #36 OR #35 OR #34 OR #33 OR #32 Indexes=SCI‐EXPANDED, SSCI, A&HCI, CPCI‐S, CPCI‐SSH Timespan=All years |
| # 37 | TI=trial Indexes=SCI‐EXPANDED, SSCI, A&HCI, CPCI‐S, CPCI‐SSH Timespan=All years |
| # 36 | TS=((singl* or doubl* or trebl* or tripl*) NEAR/1 (mask* or blind* or dumm*)) Indexes=SCI‐EXPANDED, SSCI, A&HCI, CPCI‐S, CPCI‐SSH Timespan=All years |
| # 35 | TS=(randomised or randomized or randomly or RCT or RCTs or placebo*) Indexes=SCI‐EXPANDED, SSCI, A&HCI, CPCI‐S, CPCI‐SSH Timespan=All years |
| # 34 | TS="clinical trials as topic" Indexes=SCI‐EXPANDED, SSCI, A&HCI, CPCI‐S, CPCI‐SSH Timespan=All years |
| # 33 | TS=("randomized controlled trial" or "randomised controlled trial") Indexes=SCI‐EXPANDED, SSCI, A&HCI, CPCI‐S, CPCI‐SSH Timespan=All years |
| # 32 | TS="controlled clinical trial" Indexes=SCI‐EXPANDED, SSCI, A&HCI, CPCI‐S, CPCI‐SSH Timespan=All years |
| # 31 | #30 OR #1 Indexes=SCI‐EXPANDED, SSCI, A&HCI, CPCI‐S, CPCI‐SSH Timespan=All years |
| # 30 | #29 AND #21 Indexes=SCI‐EXPANDED, SSCI, A&HCI, CPCI‐S, CPCI‐SSH Timespan=All years |
| # 29 | #28 OR #27 OR #26 OR #25 OR #24 OR #23 OR #22 Indexes=SCI‐EXPANDED, SSCI, A&HCI, CPCI‐S, CPCI‐SSH Timespan=All years |
| # 28 | TS=hallucinat* Indexes=SCI‐EXPANDED, SSCI, A&HCI, CPCI‐S, CPCI‐SSH Timespan=All years |
| # 27 | TS=(clouded near/1 state*) Indexes=SCI‐EXPANDED, SSCI, A&HCI, CPCI‐S, CPCI‐SSH Timespan=All years |
| # 26 | TS=(cloud* NEAR/3 consciousness*) Indexes=SCI‐EXPANDED, SSCI, A&HCI, CPCI‐S, CPCI‐SSH Timespan=All years |
| # 25 | TS=("acute brain" NEAR/1 (dysfunction* or failure* or syndrome*)) Indexes=SCI‐EXPANDED, SSCI, A&HCI, CPCI‐S, CPCI‐SSH Timespan=All years |
| # 24 | TS=(psychos* NEAR/3 (toxic* or exogenous* or chemical* or drug or drugs or medication* or substance*)) Indexes=SCI‐EXPANDED, SSCI, A&HCI, CPCI‐S, CPCI‐SSH Timespan=All years |
| # 23 | TS=deliri* Indexes=SCI‐EXPANDED, SSCI, A&HCI, CPCI‐S, CPCI‐SSH Timespan=All years |
| # 22 | TS=delirium Indexes=SCI‐EXPANDED, SSCI, A&HCI, CPCI‐S, CPCI‐SSH Timespan=All years |
| # 21 | #20 OR #19 OR #18 OR #17 OR #16 OR #15 OR #14 OR #13 OR #12 OR #11 OR #10 OR #9 OR #8 OR #7 OR #6 OR #5 OR #4 OR #3 OR #2 Indexes=SCI‐EXPANDED, SSCI, A&HCI, CPCI‐S, CPCI‐SSH Timespan=All years |
| # 20 | TS=(critical* NEAR/1 (ill or illness*)) Indexes=SCI‐EXPANDED, SSCI, A&HCI, CPCI‐S, CPCI‐SSH Timespan=All years |
| # 19 | TS="Critical Illness" Indexes=SCI‐EXPANDED, SSCI, A&HCI, CPCI‐S, CPCI‐SSH Timespan=All years |
| # 18 | TS=(postoperati* or post‐operati* or postsurg* or post‐surg*) Indexes=SCI‐EXPANDED, SSCI, A&HCI, CPCI‐S, CPCI‐SSH Timespan=All years |
| # 17 | TS="Postoperative Complications" Indexes=SCI‐EXPANDED, SSCI, A&HCI, CPCI‐S, CPCI‐SSH Timespan=All years |
| # 16 | TS="Postoperative Care" Indexes=SCI‐EXPANDED, SSCI, A&HCI, CPCI‐S, CPCI‐SSH Timespan=All years |
| # 15 | TS=((surgical* or surger*) NEAR/3 (unit or units or centre or centres or center or centers)) Indexes=SCI‐EXPANDED, SSCI, A&HCI, CPCI‐S, CPCI‐SSH Timespan=All years |
| # 14 | TS=(respiratory NEAR/3 (unit or units or centre or centres or center or centers)) Indexes=SCI‐EXPANDED, SSCI, A&HCI, CPCI‐S, CPCI‐SSH Timespan=All years |
| # 13 | TS=((cardiac or coronary or heart) NEAR/3 (unit or units or centre or centres or center or centers)) Indexes=SCI‐EXPANDED, SSCI, A&HCI, CPCI‐S, CPCI‐SSH Timespan=All years |
| # 12 | TS=((burn or burns) NEAR/3 (unit or units or centre or centres or center or centers)) Indexes=SCI‐EXPANDED, SSCI, A&HCI, CPCI‐S, CPCI‐SSH Timespan=All years |
| # 11 | TS=(ICU or ICUs or NICU or NICUs or PICU or PICUs or SICU or SICUs or CCU or CCUs) Indexes=SCI‐EXPANDED, SSCI, A&HCI, CPCI‐S, CPCI‐SSH Timespan=All years |
| # 10 | TS=((intensive or critical or acute) NEAR/3 care) Indexes=SCI‐EXPANDED, SSCI, A&HCI, CPCI‐S, CPCI‐SSH Timespan=All years |
| # 9 | TS="Intensive Care" Indexes=SCI‐EXPANDED, SSCI, A&HCI, CPCI‐S, CPCI‐SSH Timespan=All years |
| # 8 | TS="Critical Care" Indexes=SCI‐EXPANDED, SSCI, A&HCI, CPCI‐S, CPCI‐SSH Timespan=All years |
| # 7 | TS="Intensive Care Units, Neonatal" Indexes=SCI‐EXPANDED, SSCI, A&HCI, CPCI‐S, CPCI‐SSH Timespan=All years |
| # 6 | TS="Intensive Care Units, Pediatric" Indexes=SCI‐EXPANDED, SSCI, A&HCI, CPCI‐S, CPCI‐SSH Timespan=All years |
| # 5 | TS="Respiratory Care Units" Indexes=SCI‐EXPANDED, SSCI, A&HCI, CPCI‐S, CPCI‐SSH Timespan=All years |
| # 4 | TS="Coronary Care Units" Indexes=SCI‐EXPANDED, SSCI, A&HCI, CPCI‐S, CPCI‐SSH Timespan=All years |
| # 3 | TS="Burn Units" Indexes=SCI‐EXPANDED, SSCI, A&HCI, CPCI‐S, CPCI‐SSH Timespan=All years |
| # 2 | TS="Intensive Care Units" Indexes=SCI‐EXPANDED, SSCI, A&HCI, CPCI‐S, CPCI‐SSH Timespan=All years |
| # 1 | TS=((postoperati* or post‐operati* or postsurg* or post‐surg*) NEAR/1 ("cognitive dysfunction" or "brain dysfunction")) Indexes=SCI‐EXPANDED, SSCI, A&HCI, CPCI‐S, CPCI‐SSH Timespan=All years |
Data and analyses
Comparison 1. Duration of delirium (log ratio of means scale).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Duration of delirium (log units) | 11 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.1 Typical AP vs placebo | 4 | 608 | Mean Difference (IV, Random, 95% CI) | 0.02 [‐0.09, 0.13] |
| 1.2 Atypical AP vs placebo | 4 | 500 | Mean Difference (IV, Random, 95% CI) | ‐0.31 [‐0.71, 0.10] |
| 1.3 Statin vs placebo | 2 | 414 | Mean Difference (IV, Random, 95% CI) | 0.07 [‐0.09, 0.22] |
| 1.4 Alpha‐2 agonist vs placebo | 1 | 71 | Mean Difference (IV, Random, 95% CI) | ‐0.55 [‐0.85, ‐0.24] |
| 1.5 Cholinesterase inhibitor vs placebo | 1 | 104 | Mean Difference (IV, Random, 95% CI) | 0.61 [0.22, 0.99] |
| 1.6 Typical AP vs atypical AP | 2 | 447 | Mean Difference (IV, Random, 95% CI) | 0.05 [‐0.07, 0.17] |
| 1.7 Typical AP vs opioid | 1 | 53 | Mean Difference (IV, Random, 95% CI) | 0.09 [‐0.17, 0.34] |
Comparison 2. Delirium‐free and coma‐free days (log ratio of means scale).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Delirium‐free and coma‐free days | 4 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.1 Typical AP vs placebo | 3 | 588 | Mean Difference (IV, Random, 95% CI) | 0.13 [‐0.08, 0.34] |
| 1.2 Atypical AP vs placebo | 2 | 440 | Mean Difference (IV, Random, 95% CI) | 0.36 [‐0.15, 0.87] |
| 1.3 Statin vs placebo | 1 | 142 | Mean Difference (IV, Random, 95% CI) | ‐0.11 [‐0.33, 0.11] |
| 1.4 Typical AP vs atypical AP | 2 | 447 | Mean Difference (IV, Random, 95% CI) | ‐0.08 [‐0.21, 0.05] |
Comparison 3. Days with coma (log ratio of means scale).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Days with coma (log units) | 5 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.1 Typical AP vs placebo | 3 | 588 | Mean Difference (IV, Random, 95% CI) | ‐0.29 [‐0.71, 0.12] |
| 1.2 Atypical AP vs placebo | 2 | 440 | Mean Difference (IV, Random, 95% CI) | 0.06 [‐0.13, 0.26] |
| 1.3 Statin vs placebo | 2 | 414 | Mean Difference (IV, Random, 95% CI) | ‐0.10 [‐0.32, 0.11] |
| 1.4 Typical AP vs atypical AP | 2 | 447 | Mean Difference (IV, Random, 95% CI) | ‐0.15 [‐0.34, 0.04] |
Comparison 4. Duration of mechanical ventilation (log ratio of means scale).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Duration of mechanical ventilation (log units) | 7 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.1 Typical AP vs placebo | 3 | 515 | Mean Difference (IV, Random, 95% CI) | ‐0.08 [‐0.23, 0.06] |
| 1.2 Atypical AP vs placebo | 3 | 476 | Mean Difference (IV, Random, 95% CI) | ‐0.02 [‐0.17, 0.14] |
| 1.3 Statin vs placebo | 1 | 272 | Mean Difference (IV, Random, 95% CI) | 0.09 [‐0.11, 0.29] |
| 1.4 Alpha‐2 agonist vs placebo | 1 | 71 | Mean Difference (IV, Random, 95% CI) | ‐0.59 [‐0.89, ‐0.29] |
| 1.5 Typical AP vs atypical AP | 2 | 447 | Mean Difference (IV, Random, 95% CI) | ‐0.17 [‐0.67, 0.33] |
| 1.6 Typical AP vs opioid | 1 | 53 | Mean Difference (IV, Random, 95% CI) | ‐0.06 [‐0.37, 0.24] |
Comparison 5. Length of ICU stay (log ratio of means scale).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Length of ICU stay (log units) | 10 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.1 Typical AP vs placebo | 4 | 618 | Mean Difference (IV, Random, 95% CI) | 0.01 [‐0.11, 0.13] |
| 1.2 Atypical AP vs placebo | 4 | 577 | Mean Difference (IV, Random, 95% CI) | ‐0.09 [‐0.18, ‐0.00] |
| 1.3 Statin vs placebo | 1 | 272 | Mean Difference (IV, Random, 95% CI) | 0.06 [‐0.09, 0.21] |
| 1.4 Alpha‐2 agonist vs placebo | 1 | 71 | Mean Difference (IV, Random, 95% CI) | ‐0.22 [‐0.53, 0.08] |
| 1.5 Cholinesterase inhibitor vs placebo | 1 | 104 | Mean Difference (IV, Random, 95% CI) | 0.78 [0.46, 1.11] |
| 1.6 Typical AP vs atypical AP | 2 | 447 | Mean Difference (IV, Random, 95% CI) | 0.01 [‐0.13, 0.16] |
| 1.7 Typical AP vs opioid | 1 | 53 | Mean Difference (IV, Random, 95% CI) | 0.07 [‐0.24, 0.37] |
Comparison 6. Length of hospital stay (log ratio of means scale).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Length of hospital stay (log units) | 9 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.1 Typical AP vs placebo | 2 | 479 | Mean Difference (IV, Random, 95% CI) | ‐0.12 [‐0.38, 0.14] |
| 1.2 Atypical AP vs placebo | 3 | 511 | Mean Difference (IV, Random, 95% CI) | ‐0.04 [‐0.13, 0.05] |
| 1.3 Statin vs placebo | 2 | 369 | Mean Difference (IV, Random, 95% CI) | ‐0.01 [‐0.13, 0.12] |
| 1.4 Alpha‐2 agonist vs placebo | 1 | 71 | Mean Difference (IV, Random, 95% CI) | 0.09 [‐0.17, 0.35] |
| 1.5 Cholinesterase Inhibitor vs placebo | 1 | 104 | Mean Difference (IV, Random, 95% CI) | 0.11 [‐0.15, 0.36] |
| 1.6 Typical AP vs atypical AP | 1 | 382 | Mean Difference (IV, Random, 95% CI) | 0.04 [‐0.09, 0.17] |
| 1.7 Typical AP vs opioid | 1 | 53 | Mean Difference (IV, Random, 95% CI) | ‐0.06 [‐0.26, 0.13] |
Comparison 7. Mortality.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mortality | 10 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Typical AP vs placebo | 4 | 656 | Odds Ratio (M‐H, Random, 95% CI) | 0.98 [0.69, 1.40] |
| 1.2 Atypical AP vs placebo | 4 | 577 | Odds Ratio (M‐H, Random, 95% CI) | 1.00 [0.66, 1.52] |
| 1.3 Cholinesterase inhibitor vs placebo | 1 | 104 | Odds Ratio (M‐H, Random, 95% CI) | 1.77 [0.74, 4.26] |
| 1.4 Statin vs placebo | 2 | 414 | Odds Ratio (M‐H, Random, 95% CI) | 1.42 [0.84, 2.39] |
| 1.5 Typical AP vs opioid | 1 | 53 | Odds Ratio (M‐H, Random, 95% CI) | 2.17 [0.18, 25.46] |
| 1.6 Typical AP vs atypical AP | 2 | 447 | Odds Ratio (M‐H, Random, 95% CI) | 0.90 [0.59, 1.39] |
Comparison 8. Akathisia.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Akathisia | 2 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Typical AP vs placebo | 2 | 212 | Odds Ratio (M‐H, Random, 95% CI) | 1.34 [0.49, 3.67] |
| 1.2 Atypical AP vs placebo | 1 | 66 | Odds Ratio (M‐H, Random, 95% CI) | 1.04 [0.31, 3.50] |
| 1.3 Typical AP vs atypical AP | 1 | 65 | Odds Ratio (M‐H, Random, 95% CI) | 1.6 [0.50, 5.09] |
Comparison 9. Arrhythmias.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Arrhythmias | 4 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Typical AP vs placebo | 3 | 588 | Odds Ratio (M‐H, Random, 95% CI) | 3.09 [1.11, 8.62] |
| 1.2 Atypical AP vs placebo | 2 | 440 | Odds Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 1.3 Alpha‐2 agonist vs typical AP | 1 | 20 | Odds Ratio (M‐H, Random, 95% CI) | 1.0 [0.11, 8.95] |
| 1.4 Typical AP vs atypical AP | 2 | 447 | Odds Ratio (M‐H, Random, 95% CI) | 5.0 [0.24, 104.84] |
Comparison 10. QTc prolongation.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 QTc prolongation | 8 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Typical AP vs placebo | 4 | 656 | Odds Ratio (M‐H, Random, 95% CI) | 1.26 [0.68, 2.34] |
| 1.2 Atypical AP vs placebo | 4 | 577 | Odds Ratio (M‐H, Random, 95% CI) | 1.28 [0.45, 3.66] |
| 1.3 Typical AP vs atypical AP | 2 | 447 | Odds Ratio (M‐H, Random, 95% CI) | 0.55 [0.28, 1.08] |
| 1.4 Alpha‐2 agonist vs typical AP | 1 | 20 | Odds Ratio (M‐H, Random, 95% CI) | 1.0 [0.17, 5.98] |
| 1.5 Alpha‐2 agonist vs 5HT3 inhibitor | 1 | 64 | Odds Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 11. Extrapyramidal symptoms.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Extrapyramidal symptoms | 6 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Typical AP vs placebo | 4 | 656 | Odds Ratio (M‐H, Random, 95% CI) | 0.75 [0.26, 2.21] |
| 1.2 Atypical AP vs placebo | 3 | 476 | Odds Ratio (M‐H, Random, 95% CI) | 0.47 [0.11, 1.97] |
| 1.3 Typical AP vs atypical AP | 3 | 520 | Odds Ratio (M‐H, Random, 95% CI) | 2.22 [0.59, 8.38] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Al‐Qadheeb 2016.
| Methods |
RCT comparing the efficacy and safety of low‐dose haloperidol vs placebo for prevention of conversion of subsyndromal delirium to delirium Study took place in 3 ICUs (2 medical and 1 surgical) at a single academic medical centre in the USA |
|
| Participants |
Participants included 68 critically ill patients diagnosed with subsyndromal delirium (ICDSC score 1 to 3) (N = 34 haloperidol, mean age 61.7 ± 16.9 years, 18/34 (52.9%) male; N = 34 placebo, mean age 59.3 ± 14.9 years, 20/34 (58.8%) male) who were mechanically ventilated Study enrolment between September 2010 and August 2013 |
|
| Interventions |
Participants received 1 mg intravenous haloperidol or placebo every 6 hours until the occurrence of delirium (ICDSC score ≥ 4), a maximum of 10 days of treatment, discharge from the ICU, or an adverse effect necessitating study drug discontinuation Each dose of the study drug was administered by the bedside nurse as a slow intravenous push over 1 minute into a preexisting IV catheter and then was flushed with 10 mL of D5W. All other decisions regarding sedation, analgesia, and ventilation were left to the discretion of the ICU team Assessment: delirium status was determined based on the previous 24 hours of nursing assessments using the SAS and ICDSC Non‐drug strategies: an early mobilization protocol was implemented in 1 of 3 ICUs part‐way through the study (% ever receiving early mobilization was low). All patients were managed with the same daily awakening spontaneous breathing trial protocol |
|
| Outcomes |
Primary (measured during study drug administration)
Secondary delirium outcomes (measured during ICU admission)
|
|
| Notes |
Funding
Registration
Study authors were not contacted |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomized in blocks of 4 in a 1:1 ratio by means of a computer‐generated random numbers table. Treatment allocation was known only to the investigational pharmacist |
| Allocation concealment (selection bias) | Low risk | States treatment allocation was known only to the investigational pharmacist. Electronic randomization was performed |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Treatment allocation was known only to the investigational pharmacist. Participants, clinicians, and all study personnel were blinded to study drug assignment. Each study dose was prepared by the investigational pharmacy so an identical looking 0.5‐mL tuberculin syringe contained 0.2 mL of haloperidol 1 mg or 5% dextrose |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Clinicians, investigators, participants, and their families remained blinded to treatment allocation. A study investigator confirmed the presence of delirium with the bedside nurse using the ICDSC assessment. The presence of delirium was subsequently confirmed by a consulting psychiatrist. Discordance between the psychiatric consultation and the bedside nurse and study investigator’s ICDSC assessments was resolved through consensus |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All randomized participants were included in the analysis. Data were analysed according to an intention‐to‐treat approach |
| Selective reporting (reporting bias) | Low risk | All outcomes reported in methods were included in results. Data were presented for all participants included in the analysis |
| Other bias | Low risk | Use of dexmedetomidine and non‐study antipsychotic drugs was not permitted unless deemed medically necessary An early mobilization protocol was implemented in 1 of 3 ICUs part‐way through the study (% ever receiving early mobilization was low). All participants were managed with the same daily awakening spontaneous breathing trial protocol Sample size calculation was provided |
Atalan 2013.
| Methods |
RCT comparing the efficacy of haloperidol vs morphine for treatment of postoperative delirium Study took place in a single ICU at a community hospital in Turkey |
|
| Participants |
Participants included 53 (N = 26 haloperidol, mean age 66.00 ± 8.39 years, 21/26 (80.8%) male; N = 27 morphine, mean age 65.74 ± 9.67 years, 18/27 (66.7%) male) patients who underwent cardiac surgery, with or without cardiopulmonary bypass, and were diagnosed with hyperactive delirium using the CAM‐ICU and RASS (to determine subtype) Study enrolment between January 2010 and July 2012 |
|
| Interventions |
Participants received 5 mg haloperidol or 5 mg morphine sulphate intramuscularly every hour until adequate sedation (RASS ‐1 to + 1) was achieved. Participants who were still agitated despite administration of 20 mg/d morphine or 20 mg/d haloperidol received 2.5 mg lorazepam orally, twice daily Assessment: delirium status (CAM‐ICU) was determined every 12 hours until discharge from hospital or for a maximum of 10 days following surgery. Participants were considered delirium‐free after a period of 24 hours without symptoms Non‐drug strategies: not reported |
|
| Outcomes |
Primary (measured at completion of study drug)
Secondary (measured at completion of study drug)
|
|
| Notes |
Funding
Registration
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The manuscript stated that participants were randomized to 2 groups but provided no specific details on the method used |
| Allocation concealment (selection bias) | Unclear risk | No details were provided on the method of randomization or concealment used. Attempts to obtain details from study authors were not successful |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No details were provided on the method of drug preparation and dispensing used. Attemtps to obtain details from study authors were not successful |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Abnormal or delirious behaviour was recorded by the bedside nurse and reviewed by the research team. Clinical evaluation was performed by the intensivist and the consulting psychiatrist, who were blinded to study group assignment |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No flow chart was included to report the numbers of screened vs randomized participants |
| Selective reporting (reporting bias) | Unclear risk | All outcomes reported in methods were included in results. Data presented for all participants were included in the analysis. However without a published protocol or trial registration, it is unknown if all outcomes were reported as planned |
| Other bias | Unclear risk | The funding source for the study was not mentioned All participants were permitted rescue lorazepam Sample size calculation was not provided |
Bakri 2015.
| Methods |
RCT comparing the efficacy of dexmedetomidine, ondansetron, and haloperidol for treatment of postoperative delirium Study took place in a single 24‐bed ICU (mixed medical and surgical) in Saudi Arabia |
|
| Participants |
Participants included 96 (N = 32 dexmedetomidine, mean age 31 ± 4 years, 29/32 (91% male); N = 32 ondansetron, mean age 32 ± 5 years, 30/32 (94%) male, N = 32 haloperidol, mean age 30 ± 7 years, 28/32 (88%) male) critically ill trauma patients diagnosed with postoperative delirium (ICDSC score ≥ 4) Study enrolment between 2011 and 2013 |
|
| Interventions |
Participants received 1 μg/kg dexmedetomidine, 4 mg ondansetron, or 5 mg haloperidol, administered as a continuous intravenous infusion over 20 minutes. Study drug was started after delirium was diagnosed and was given twice daily for 3 consecutive days Treating physicians were allowed to prescribe rescue haloperidol for all groups Assessment: ICDSC was administered twice daily Non‐drug strategies: not reported |
|
| Outcomes |
Primary outcome (measured day 3)
Secondary outcomes (measured day 3)
|
|
| Notes |
Funding
Registration
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomly allocated to 3 equal groups according to a computer‐generated random numbers sequence |
| Allocation concealment (selection bias) | Unclear risk | Method of concealment was not reported and was not available from study authors |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Study medications were prepared by physicians who were not part of the research team. All study drug was administered as a continuous infusion over a 20‐minute period to maintain blinding |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Data were collected by researchers blinded to study allocation. Participants were managed by ICU staff not included in the study. ICU nurses conducted delirium assessments as part of the standard of care |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No flow chart was included to report numbers of screened vs randomized participants |
| Selective reporting (reporting bias) | Unclear risk | All outcomes reported in methods were included in results. Data presented for all participants were included in the analysis. However without a published protocol or trial registration, it is unknown if all outcomes were reported as planned |
| Other bias | Unclear risk | All participants were permitted rescue haloperidol. Use of rescue haloperidol was an outcome Sample size calculation was not reported |
Devlin 2010.
| Methods |
RCT comparing the efficacy and safety of quetiapine vs placebo for treatment of delirium Study took place in 3 ICUs (mixed medical and surgical) at 3 academic medical centres ‐ 2 in the USA and 1 in Canada |
|
| Participants |
Participants included 36 (N = 18 quetiapine, mean age 62.4 ± 14 years, 51% male; N = 18 placebo, mean age 63.6 ± 15.3 years, 56% male) critically ill patients with diagnosis of delirium (ICDSC score ≥ 4), requiring as needed haloperidol and tolerating enteral nutrition without a complicating neurological condition Study enrolment between April 2006 and August 2008 |
|
| Interventions |
Participants received an initial dose of 50 mg quetiapine or placebo given orally or via nasogastric/enteral feeding tube. Daily titration of 50‐mg increments every 12 hours (to maximum 200 mg every 12 hours) was permitted if participant received at least 1 dose of as needed haloperidol All participants were permitted as needed intravenous haloperidol (1 to 10 mg), administered up to every 2 hours. Study drug was continued until delirium resolution (based on clinical judgement of attending intensivist), 10 days of treatment, ICU discharge, or occurrence of an adverse event attributable to study drug and warranting its discontinuation Assessment: delirium status was determined by every nursing shift using the ICDSC Non‐drug strategies: not reported |
|
| Outcomes |
Primary (measured at completion of study drug)
Secondary efficacy outcomes (measured at completion of study drug)
|
|
| Notes |
Funding
Post‐hoc analysis of the trial compared duration and time to first resolution of individual delirium symptoms from participants in the original study (Devlin 2011) Registration
Study authors were contacted for clarification and responded |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were assigned in blocks of 4 to 1 to the 2 groups in a 1:1 ratio by means of a computer‐generated random numbers table. A different randomization schedule was used at each site. Treatment allocation was known only to the investigational pharmacist at each site |
| Allocation concealment (selection bias) | Low risk | Treatment allocation was known only to the investigational pharmacist at each site. Electronic randomization was performed |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Participants and all study personnel were blinded to study drug assignment. Tablets were identical to one another, even when crushed |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Participants and all study personnel were blinded to study drug assignment. Delirium assessments were completed by ICU nurses as part of the standard of care |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data were analysed using the intention‐to‐treat principle. All randomized participants were included in the analysis |
| Selective reporting (reporting bias) | Low risk | Data presented for all participants were included in the analysis No trial registration was reported; however the original REB application was obtained from the study author |
| Other bias | Low risk | All participants were permitted rescue haloperidol Sample size calculation was reported |
Girard 2010a.
| Methods |
RCT comparing haloperidol, ziprasidone, and placebo on the number of days alive and without delirium or coma among ICU patients (49% of all patients were delirious at enrolment, and 35% were comatose) Study took place in 6 ICUs (mixed medical and surgical) at 6 tertiary care centres in the USA |
|
| Participants |
Participants included 101 (N = 35 haloperidol, median age 51 (IQR 35 to 59) years, 20/35 (57% male); N = 30 ziprasidone, median age 54 (IQR 47 to 66), 21/30 (70%) male; N = 36 placebo, median age 56 (IQR 43 to 68), 22/36 (61%) male) mechanically ventilated, critically ill patients with an abnormal level of consciousness or receiving sedative or analgesic medications Study enrolment between February 2005 and July 2007 |
|
| Interventions |
Participants received 5 mg haloperidol (as a solution containing 1 mg/mL), 40 mg ziprasidone (as a solution containing 8 mg/mL), or placebo (as a 5‐mL solution). In patients without gastric access, study drug was given via 0.5‐mL intramuscular injection (to a maximum of 8 doses). If QTc remained < 500 ms, the second dose of study drug was administered 12 hours after the first, and subsequent doses were given every 6 hours until a change in frequency was warranted. If 2 consecutive assessments for delirium/coma were negative, drug frequency was decreased to every 8 hours, and the drug was discontinued if no delirium or coma was noted for 48 hours. Study drug was reduced if patients remained over‐sedated (RASS ≥ 2 levels deeper than target score) despite discontinuation of sedatives. Study drug was restarted or increased in frequency when over‐sedation was resolved. Study drug was restarted (if discontinued prior) or increased to the previously effective dose if delirium recurred. Study drug was discontinued if extrapyramidal symptoms (≥ 3 points on 3 or more categories of the Simpson‐Angus Scale) or QTc prolongation (> 500 ms) occurred and was restarted only if these were resolved All patients stopped study drug on day 14 regardless of clinical status Other treatments including approaches to sedation were determined by the managing ICU team. Daily spontaneous awakening trials were common but were not protocolized An open‐label antipsychotic was strongly discouraged during the trial but could be used if the ICU team considered it necessary for breakthrough agitation Assessment: brain dysfunction was assessed twice daily using CAM‐ICU and RASS Non‐drug strategies: none of the ICUs used formalized non‐pharmacological interventions to prevent or treat delirium |
|
| Outcomes |
Primary
Secondary
|
|
| Notes |
Funding
Dr Girard received support from
Dr Pandharipande received support from
Dr Ely received support from
Registration
Study authors were not contacted |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomly assigned in a 1:1:1 manner via a computer‐generated, permuted block randomization scheme stratified according to study centre |
| Allocation concealment (selection bias) | Low risk | A co‐ordinating centre biostatistician designated treatment group assignments on a list that was provided only to the investigational pharmacists at each study centre, who referred to their unique list to determine group assignment after each patient was enrolled |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | This was a double‐blind study. Except for the pharmacist, neither study personnel nor participants were aware of treatment group assignment. Participants received 1 of the 3 colourless, odourless, and tasteless study drugs |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinded trained study personnel evaluated participants twice daily for acute brain dysfunction, diagnosing delirium with CAM‐ICU |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data were analysed via an intention‐to‐treat principle. 2 participants were excluded after randomization, before study drug was administered, because of ventricular tachycardia. No outcome data could be collected for these 2 participants after their withdrawal |
| Selective reporting (reporting bias) | Low risk | All outcomes reported in methods were included in results. Data presented for all participants were included in the analysis |
| Other bias | Low risk | Open‐label antipsychotic administration was strongly discouraged during the trial but could be provided if the clinical team considered it necessary for breakthrough delirium and agitation. Pfizer Inc. had no role in the design or conduct of the trial; in the collection, analysis, or interpretation of data; nor in the preparation, review, approval, or publication strategy of the study manuscript |
Girard 2018.
| Methods |
RCT comparing haloperidol, ziprasidone, and placebo on the number of days alive and without delirium or coma in ICU patients Study took place in 16 ICUs (mixed medical and surgical) in the United States |
|
| Participants | Adult (≥ 18 years) medical/surgical ICU participants on mechanical or non‐invasive positive‐pressure ventilation and/or requiring vasopressors due to shock, or an intra‐aortic balloon pump, and diagnosed with delirium by CAM‐ICU Participants included 566 (N = 189 haloperidol, median age 61 (IQR 51 to 69) years, 44% female); N = 183 ziprasidone, median age 61 (IQR 50 to 69), 43% male; N = 179 placebo, median age 59 (IQR 52 to 67), 42% female) Study enrolment between December 2011 and August 2017 To minimize the time between onset of delirium and randomization, informed consent was often obtained before the onset of delirium |
|
| Interventions |
Participants were randomized to receive haloperidol (up to 10 mg every 12 hours, administered by intravenous bolus over up to 5 minutes at concentrations of 5 mg/mL), ziprasidone (up to 20 mg every 12 hours, administered by intravenous bolus over up to 5 minutes at concentrations of 10 mg/mL), or placebo (up to 10 mg every 12 hours, administered by intravenous bolus over up to 5 minutes). Participants will be treated until delirium has resolved for 48 hours, or when 14 days of treatment have elapsed, whichever occurs first All patients stopped study drug on day 14 regardless of clinical status Open‐label antipsychotic use was permitted (21%; no differences between groups) Assessment: brain dysfunction was assessed twice daily using CAM‐ICU and RASS Non‐drug strategies: all ICUs used formalized non‐pharmacological interventions to prevent or treat delirium ‐ specifically, the ABCDE treatment bundle (assess, prevent, and manage pain; both spontaneous awakening and breathing trials; choice of analgesia and sedation; assess, prevent, and manage delirium; and early mobility and exercise). Compliance was > 88% in each study group |
|
| Outcomes |
Primary
Secondary
|
|
| Notes | MIND‐USA study: modifying the impact of ICU‐associated neurological dysfunction Funding
Registration
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomly assigned in a 1:1:1 manner via a computer‐generated, permuted block randomization scheme stratified according to study centre |
| Allocation concealment (selection bias) | Low risk | A co‐ordinating centre biostatistician designated treatment group assignments on a list that was provided only to the investigational pharmacists at each study centre, who referred to their unique list to determine group assignment after each patient was enrolled |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | This was a double‐blind study. Except for the pharmacist, neither study personnel nor participants were aware of treatment group assignment. Study drugs were identical colourless preparations |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinded trained study personnel evaluated participants twice daily for acute brain dysfunction, diagnosing delirium using CAM‐ICU |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All outcomes reported in methods were included in results. Data presented for all participants were included in the analysis |
| Selective reporting (reporting bias) | Low risk | All outcomes reported in methods were included in results. Data presented for all participants were included in the analysis. The trial was registered in advance. The statistical plan was registered at Open Science Framework (https://osf.io/mq38r) before the trial group assignments were unmasked |
| Other bias | Low risk | Open‐label antipsychotic administration was strongly discouraged during the trial but could be used if the clinical team considered it necessary for breakthrough delirium and agitation. There was no difference between study groups in the use of open‐label antipsychotics |
Hakim 2012.
| Methods |
RCT comparing risperidone and placebo for prevention of conversion of subsyndromal delirium to delirium Study took place in a single cardiosurgical ICU in Egypt |
|
| Participants |
Participants included 101 elderly (aged ≥ 65) (N = 51 risperidone, mean age not provided, 33/51 male; N = 50 placebo, mean age not provided, 36/50 male) on‐pump cardiac surgery patients with diagnosis of postsurgical subsyndromal delirium (ICDSC score 1 to 3) Study enrolment between December 2007 and November 2010 |
|
| Interventions | Participants with subsyndromal delirium (ICDSC score 1 to 3) received oral 0.5 mg risperidone or placebo every 12 hours until 24 hours after subsidence of subsyndromal delirium (ICDSC score of 0) or development of frank delirium (ICDSC ≥ 4). Among delirious participants, treatment allocation was revealed, and placebo‐treated patients were started on 0.5 mg oral risperidone every 12 hours. If symptoms remained uncontrolled, the dose was increased to a maximum of 4 mg/d. Among delirious risperidone‐treated patients, the dose was increased until symptoms were controlled or a maximum dose of 4 mg/d was attained. Haloperidol was used in both groups if symptoms were not controlled with maximal risperidone dose. Haloperidol was started at 0.5 mg every 8 hours and could be increased to 10 mg/d if needed. Haloperidol dose could be doubled every 24 hours until symptoms were controlled or the maximum dosage was attained Rescue medications were continued for 24 hours after a score of 0 was achieved on the ICDSC Assessment:screening for subsyndromal delirium was done using the ICDSC, began 4 hours after extubation in the ICU, and was continued for every 8‐hour nursing shift thereafter, including after discharge to the cardiosurgical ward Non‐drug strategies: not reported |
|
| Outcomes |
Primary (end of study)
Secondary (end of study)
|
|
| Notes |
Funding
Registration
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomized by a clinical pharmacist in a 1:1 ratio via a computer‐generated random numbers list created with GraphPad StatMate v.1.01i software (Graph‐Pad Software Inc., San Diego, CA, USA) using permuted blocks of size 4 |
| Allocation concealment (selection bias) | Low risk | Allocation group codes were typed and were kept at the pharmacy in sealed envelopes. Treatment concealment was maintained until recruitment, data collection, and analysis were completed, unless an emergency warranted otherwise and was requested by an attending physician |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | This was a double‐blind study. Test drugs were prepared by the hospital’s pharmacy and were identical in appearance and odour. Drugs were dispensed in identical containers sealed and numbered according to the random number list |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | 4 intensivists and 3 ward physicians, who were blinded to group allocation, were charged with screening participants for subsyndromal delirium using the ICDSC. Randomized participants were assessed by a blinded observer using the ICDSC, and those scoring > 3 were evaluated by a blinded psychiatrist to confirm delirium |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data were analysed using an intention‐to‐treat principle. All randomized participants were included in the analysis |
| Selective reporting (reporting bias) | Unclear risk | All outcomes reported in methods were included in results. Data presented for all participants were included in the analysis. However without a published protocol or trial registration, it is unknown if all outcomes were reported as planned |
| Other bias | Low risk | All participants were permitted rescue haloperidol Sample size calculation was provided |
Needham 2016.
| Methods |
Ancillary study to an RCT of rosuvastatin vs placebo for delirium Study took place in 35 ICUs (mixed medical and surgical) in the USA |
|
| Participants |
Participants included 272 (N = 137 rosuvastatin, mean age 52 ± 18 years, 72/137 (53%) male; N = 135 placebo, mean age 52 ± 16 years, 65/135 (48%) male) adult ICU patients meeting criteria for acute respiratory distress syndrome, receiving mechanical ventilation through an endotracheal tube, and meeting criteria for systemic inflammatory response with a known or suspected infection Study enrolment between Janurary 2010 and November 2013 |
|
| Interventions |
Participants received a 40‐mg loading dose of rosuvastatin (and a daily 20‐mg dose) or placebo at randomization until 3 days after discharge from intensive care, study day 28, or death, whichever occurred first Delirium was assessed daily by clinical or research personnel using the CAM‐ICU |
|
| Outcomes |
Primary
Secondary
|
|
| Notes | Funding was provided by:
The funders had no role in study design; collection, analysis, and interpretation of data; writing of the report; or the decision to submit for publication This is an ancillary study of the SAILS trial (https://clinicaltrials.gov/ct2/show/NCT00979121) ‐ a randomized controlled trial assessing mortality and ventilator‐free days for rosuvastatin vs placebo in patients with sepsis‐associated acute respiratory distress syndrome SAILS was stopped early because of futility, after recruiting 745 of 1000 patients, with no significant differences in short‐term mortality, ventilator‐free days, or intensive care unit‐free days Additional unpublished data were provided by study authors |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomly assigned in permuted blocks of 8, with stratification by hospital, via a web‐based system |
| Allocation concealment (selection bias) | Low risk | Participants were randomly assigned in permuted blocks of 8, with stratification by hospital, via a web‐based system. Each research co‐ordinator used a unique personal identification number to access the system. Treatment assignment and individual subject identification numbers were assigned. An emailed confirmation to the study site followed |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Study drug was blinded by an identical appearing placebo |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | This was a double‐blind study |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Primary analysis was done for the intention‐to‐treat population, with participants contributing to the model on days when delirium could be assessed (i.e. no coma) |
| Selective reporting (reporting bias) | Low risk | All outcomes reported in methods were included in results. Additional data were provided by study authors for this review |
| Other bias | Low risk | The funders had no role in study design; collection, analysis, and interpretation of data; writing of the report; or the decision to submit for publication |
Page 2013.
| Methods |
RCT comparing haloperidol vs placebo on the duration of delirium coma Study took place in a single ICU (mixed medical and surgical) in the UK |
|
| Participants |
Participants included 141 (N = 71 haloperidol, mean age 67.9 ± 16.5 years, 37/71 (52%) male; N = 70 placebo, mean age 68.7 ± 14.9 years, 45/70 (64%) male) critically ill patients requiring mechanical ventilation within 72 hours of ICU admission Study enrolment between November 2010 and September 2012 |
|
| Interventions |
Participants received haloperidol 2.5 mg or an equal volume of 0.9% saline, intravenously, every 8 hours Study drug was discontinued
If a patient screened positive for delirium again within the 14‐day study period, the study drug was re‐administered. Patients were kept on fentanyl and propofol infusions, titrated to a RASS of 0 to ‐1, unless deeper sedation was required. If a patient was over‐sedated, study drug dose was halved; if over‐sedation lasted longer than 24 hours, study drug was stopped. If a patient developed acute agitation (RASS +2 or higher), reversible causes were investigated and treated. If the agitation did not resolve, the patient was allowed up to 10 mg intravenous haloperidol in a 24‐hour period (2.5‐ to 5.0‐mg doses) Delirium status was assessed via the CAM‐ICU twice during each 12‐hour shift, with a minimum of 4 hours between 2 consecutive assessments. Delirium was defined if RASS was ‐2 to +4 and CAM‐ICU was positive |
|
| Outcomes |
Primary
Secondary
|
|
| Notes |
Funding
The sponsor of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report. Additional study outcomes were published in abstract form (page 2015). This analysis served to determine long‐term survival, quality of life, and cost‐effectiveness of the use of haloperidol in the original trial Registration
Study authors were contacted for clarification; they provided the requested information Erratum in Lancet Respir Med 2013 Oct;1(8):592 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A nurse from the operating theatre post‐anaesthetic care unit, who was independent of the ICU clinical and research staff, allocated participants in a 1:1 ratio using random permuted blocks of sizes 4 and 6 and a centralized, secure web‐based randomization service |
| Allocation concealment (selection bias) | Low risk | Study was randomized via a centralized, secure web‐based randomization service |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Study drugs were prepared in the PACU, which was separate from the ICU, in identical syringes by an independent member of the PACU nursing staff, who administered the drug |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | All ICU clinical and research staff, legal representatives, and participants were masked to study drug. The data monitoring and safety committee reviewed blinded data reports. Statisticians were not masked to allocation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data were analysed according to an intention‐to‐treat principle. 1 participant in the placebo group was withdrawn after failure to obtain consent to continue or use collected data; this patient’s data were not included in the final analysis |
| Selective reporting (reporting bias) | Low risk | All outcomes reported in methods were included in results. Data presented for all participants were included in the analysis |
| Other bias | Low risk | The sponsor of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report |
Page 2017.
| Methods |
RCT comparing simvastatin vs placebo on duration of delirium coma Study took place in a single ICU (mixed medical and surgical) in the UK |
|
| Participants |
Participants included 142 (N = 71 simvastatin, mean age 61.9 ± 15.3 years, 45/71 (63%) male; N = 71 placebo, mean age 62.1 ± 17.3 years, 37/71 (52%) male) critically ill patients requiring mechanical ventilation within 72 hours of ICU admission Study enrolment between February 2013 and January 2015 |
|
| Interventions |
Participants received 80 mg simvastatin or placebo within 72 hours of admission to the ICU, irrespective of the presence of coma or delirium. Study drug was given daily, orally or by feeding tube. Treatment was discontinued at ICU discharge, after a maximum of 28 days, at death, with creatine kinase concentrations > 10 times the upper limit of normal, with alanine transaminase concentrations > 8 times the upper limit of normal, with development of a clinical condition requiring immediate treatment with statins, upon discontinuation of active medical treatment, with request for discontinuation by patient or legal representative, or upon request for discontinuation by attending clinician or contraindication to enteral drug administration. Patients were kept on fentanyl and propofol infusions, titrated to a RASS of 0 to ‐1, unless deeper sedation was required Delirium status was assessed via the CAM‐ICU twice during each 12‐hour shift, with a minimum of 4 hours between 2 consecutive assessments. Delirium was defined if RASS was ‐2 to +4 and CAM‐ICU was positive |
|
| Outcomes |
Primary
Secondary
|
|
| Notes |
Funding
Sponsored by
Co‐ordinated by
Registration
Study authors were contacted for clarification, and requested information was provided Erratum in Corrections (Lancet Respir Med 2018) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The study statistician generated the randomization schedule in advance using nQuery Advisor version 4.0; randomization was done by variable block sizes of 2, 4, 6, and 8, without stratification |
| Allocation concealment (selection bias) | Low risk | No details were provided pertaining to the individual responsible for randomizing participants. Patient drug packs were prepared by Victoria Pharmaceuticals (Belfast, Northern Ireland) according to the pre‐arranged randomization schedule and were distributed to the hospital pharmacy, which stored the packs in a secure area and dispensed them to the ICU as required. Each pack was numbered with a unique patient trial identifier that had been allocated to each participant at the time of random assignment to a group |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | This was a double‐blind study. Simvastatin or placebo tablets were packaged in white opaque high‐density polyethylene plastic containers sealed with a tamper‐evident seal. Placebo and simvastatin tablets were indistinguishable when crushed and dispersed in water for enteral administration |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | All ICU clinical and research staff, legal representatives, and participants were masked to study drug. The data monitoring and safety committee reviewed blinded data reports. Statisticians were not masked to allocation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data were analysed according to an intention‐to‐treat principle. All randomized participants were included in the primary analysis |
| Selective reporting (reporting bias) | Low risk | All outcomes reported in methods were included in results. Data presented for all participants were included in the analysis |
| Other bias | Low risk | The funder of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report |
Reade 2009.
| Methods |
RCT comparing haloperidol vs dexmedetomidine in facilitating extubation for patients with severe agitation Study took place in a single 20‐bed ICU (mixed medical and surgical) at a university hospital in Australia |
|
| Participants |
Participants included 20 (N = 10 haloperidol, median age 68.5 (IQR 43 to 78) years, 80% male; N = 10 dexmedetomidine, median age 52 (IQR 42 to 69) years, 90% male) mechanically ventilated, critically ill patients who could not be extubated because their level of agitation (e.g. RASS score ≥ 2) required such a high dose of sedative drug (40% haloperidol group delirious at enrolment, 30% dexmedetomidine, using ICDSC ≥ 4; 100% haloperidol group at least subsyndromal delirium at enrolment, 80% dexmedetomidine, using ICDSC ≥ 0) Study enrolment between April 2006 and August 2008 |
|
| Interventions |
Participants received dexmedetomidine, started as an intravenous infusion of 0.2 to 0.7 mcg/kg/h (option of providing a loading dose of 1.0 μg/kg IV over a 20‐minute period), or haloperidol, started as an intravenous infusion of 0.5 to 2.0 mg/h (option of providing a loading dose of 2.5 mg). Nurses adjusted infusion rates as necessary (re‐assessing at least every 4 hours), with the aim of minimizing psychomotor agitation and achieving a RASS score of 0. Treatment was continued for as long as was deemed necessary by the treating physician, including following extubation. There was no strict protocol outlining the titration of either drug. Dexmedetomidine was not available in the hospital's formulary; once it was stopped, it could not be restarted. Haloperidol could however be administered for as long as needed The bedside nurse was responsible for transitioning the patient from mechanical to spontaneous ventilation as early as possible and through assessments done every 4 hours |
|
| Outcomes |
Primary
Secondary efficacy outcomes
Secondary safety outcomes
|
|
| Notes |
Funding was provided by
Dexmedetomidine was supplied free of charge by the manufacturer, Hospira, which had no other involvement in the study Registration
Study authors were contracted and clarifications were provided |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A computer‐generated random‐number sequence was used. Numbered envelopes contained a card indicating allocation |
| Allocation concealment (selection bias) | Low risk | Participants were allocated via numbered envelopes into which a card indicating patient allocation had been placed according to a computer‐generated random numbers sequence |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | The study used an open‐label study design. Clinical personnel were not blinded to the study drug |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | The study used an open‐label study design and was not blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All randomized participants were included in the analysis. No eligible participants' relatives refused consent, and no patients were lost to follow‐up. 1 participant in the haloperidol group stopped the drug at physician request |
| Selective reporting (reporting bias) | Low risk | All outcomes reported in methods were included in results. Data presented for all participants were included in the analysis |
| Other bias | Low risk | Dexmedetomidine was supplied free of charge by the manufacturer, Hospira, which had no other involvement in the study |
Reade 2016.
| Methods |
RCT comparing dexmedetomidine vs placebo in facilitating extubation for patients with agitated delirium Study took place in 15 ICUs (14 mixed medical‐surgical and 1 primarily cardiac postoperative) in Australia and New Zealand |
|
| Participants |
Participants included 71 (N = 39 dexmedetomidine, median age 58 (IQR 47 to 65) years, 28/39 (71.8%) male; N = 32 placebo, median age 56.5 (IQR 46 to 69.5) years, 25/32 (78.1%) male) patients who required continued mechanical ventilation because their level of agitation was so severe that reducing sedation or extubation was deemed unsafe Participants met the following criteria during the 4 hours before randomization
Study enrolment between May 2011 and December 2013 |
|
| Interventions | Participants received intravenous dexmedetomidine or placebo (saline), started at a dose of 0.5 μg/kg/h (option of 1.0 μg/kg bolus over 20 minutes). Study drug was titrated by the bedside nurse between 0 and 1.5 μg/kg/h to achieve RASS score of 0 or to physician‐prescribed target. After 48 hours of study drug infusion, the treating physician could prescribe open‐label dexmedetomidine and the study drug was stopped. More than 7 days of infusion of study drug was considered treatment failure, at which point study drug was stopped and open‐label dexmedetomidine was started | |
| Outcomes |
Primary
Secondary
|
|
| Notes |
Funding
The sponsoring pharmaceutical company (Hospira Australia) decided against extending funding and provision of study drug beyond a date that had been earlier agreed. Consequently, the trial was terminated prematurely in December 2013, after 74 patients had been randomized. The funders had no role in the design and conduct of the study; collection, management, analysis, and interpretation of data; preparation or approval of the manuscript; or decision to submit the manuscript for publication Registration
Study authors were contacted for clarification and provided requested details Erratum in Expanded Explanation of the Sample Size Calcualtion (JAMA 2016) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomized, stratified by site and age (< 55 years and ≥ 55 years), in concealed permuted blocks of 2 to 6 by a computer‐generated algorithm accessed via Internet connection to the Australian and New Zealand Research Centre at Monash University |
| Allocation concealment (selection bias) | Low risk | Participants were randomized in concealed permuted blocks of 2 to 6 by a computer‐generated algorithm accessed via Internet connection to the Australian and New Zealand Research Centre at Monash University. Unblinded pharmacists or nurses not involved in the care of study participants prepared study drug in identically labelled syringes |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | This was a double‐blind study. Participants randomized to placebo received an identically labelled infusion of saline at an equivalent rate. Physicians and nurses treating study participants and the study staff at each site remained blinded to group allocation |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Frequency of delirium screening was not mentioned. Bedside nurses performed delirium assessments. The decision to extubate was determined by senior ICU physicians, taking into account assessments of bedside nurses. This decision was not part of the protocol but instead was tailored to individual patient circumstances, with a physician constantly present at each ICU |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data were analysed through modified intention‐to‐treat analyses. Modification was permitted to account for post‐randomization circumstances that prevented use of data from certain participants. Because no data for the primary outcome were missing and less than 5% was missing for all secondary outcomes, no data imputation was performed |
| Selective reporting (reporting bias) | Low risk | All outcomes reported in methods were included in results. Data presented for all participants were included in the analysis |
| Other bias | Low risk | The funders had no role in the design and conduct of the study; collection, management, analysis, and interpretation of data; preparation or approval of the manuscript; or the decision to submit the manuscript for publication |
Skrobik 2004.
| Methods |
RCT comparing olanzapine vs haloperidol for treatment of delirium Study took place: single 16‐bed tertiary care university‐affiliated ICU (mixed medical‐surgical) in Canada |
|
| Participants |
Participants included 73 (N = 45 haloperidol, mean age 63.26 ± 11.66 years, 31/45 male; N = 28 olanzapine, mean age 67.50 ± 6.04 years, 22/28 male) critically ill patients admitted to the ICU for longer than 24 hours and diagnosed with delirium (ICDSC ≥ 4, confirmed by DSM‐IV criteria) Study enrolment July 2000 to September 2001 |
|
| Interventions |
The intensivist prescribed haloperidol or olanzapine PO (or via feeding tube, if necessary) within 2 hours of delirium diagnosis. Haloperidol was initiated at 2.5 to 5 mg every 8 hours, and olanzapine was begun at 5 mg daily. Patients over 60 years of age received a lower initial dosage (haloperidol 0.5 to 1 mg, or olanzapine 2.5 mg). Subsequent titration was based on clinical judgement. Clinicians and nurses titrated sedatives to targeted RASS score, and use of rescue IV haloperidol was left to the discretion of the treating intensivist The Delirium Index (DI) was administered by 1 of 2 research nurses and a physician at baseline and daily for up to 5 days |
|
| Outcomes |
|
|
| Notes |
Funding
Registration No trial registration number was included in the manuscript Study authors were contacted for clarifications and provided requested information |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Randomization was performed on an even/odd day basis |
| Allocation concealment (selection bias) | High risk | Method of allocation concealment was not reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Treating physicians and nurses were not blinded to the assigned drug |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Objective evaluations were performed on a daily basis by a clinician or a research nurse blinded to the dispensed medication |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Informed consent was obtained for 80 participants; of these, the treating physician withdrew 3 participants, status was changed to “no active treatment” for 2, drug interaction was suspected for 1, and data for 1 were lost. 73 participants were included in the final analysis |
| Selective reporting (reporting bias) | Unclear risk | Published protocol or trial registration was not available |
| Other bias | Low risk | Participants who developed agitation during the study were permitted intravenous haloperidol administration (recorded as “rescue haloperidol”) |
van Eijk 2010.
| Methods |
RCT comparing rivastigmine vs placebo as an adjunct to haloperidol for treatment of delirium Study took place in 6 ICUs in the Netherlands |
|
| Participants |
Participants included 104 (N = 54 rivastigmine, mean age 68.0 ± 11.4 years, 38/54 (70%) male; N = 50 placebo, mean age 70.0 ± 12.2 years, 29/50 (58%) male) critically ill patients diagnosed with delirium according to the CAM‐ICU and expected to remain in the ICU for at least 48 hours Study enrolment between November 2008 and January 2010 |
|
| Interventions |
Participants received rivastigmine or placebo twice daily. Rivastigmine was delivered in a 2‐mg/mL solution The dosing regimen for rivastigmine was as follows
Once delirium was resolved or participants were discharged from hospital, the dose regimen was reversed and study drug was tapered off over 3 days. If a possible side effect occurred during treatment, study drug was reduced until the side effect was resolved, or was stopped if the side effect persisted for longer than 3 days. Participants with persistent side effects were followed until an endpoint was reached (end of delirium, discharge from hospital, or death) Delirium was assessed daily using the CAM‐ICU until 3 days after study drug cessation. The CAM was used if the patient was discharged to a regular ward. All participants received usual care including frequent orientation, physical therapy, and exercise. Participants ≥ 70 years of age received 1 mg intravenous haloperidol 3x/d, and those aged ≤ 69 years received 2.5 mg intravenous haloperidol 3x/d. Participants were allowed to receive 1 mg intravenous lorazepam at night (22:00). The treating physician could adjust treatment with haloperidol or a benzodiazepine, similar to usual care. Rescue haloperidol (2.5 mg if ≥ 70 years, and 5 mg ≤ 69 years) was recommended in the event of persistent agitation and was repeated every 30 minutes if needed. If haloperidol proved ineffective, 1 mg/kg per hour intravenous propofol was administered. In the event that propofol was contraindicated, 5 mg per hour intravenous midazolam was used instead. The dose of propofol or midazolam was increased until the participant was calm but was tapered every 12 hours thereafter. Study drug was continued during sedation |
|
| Outcomes |
Primary
Secondary
Other outcomes
|
|
| Notes |
Funding
None of the funding sources had any role in the design or conduct of the study Registration
Study authors were not contacted |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomized in a 1:1 ratio. The randomization sequence was computer generated by the trial pharmacist and was stratified by study centre. The leading pharmacist held a list of study codes, which could be broken at any time if deemed necessary by the treating physician |
| Allocation concealment (selection bias) | Low risk | The trial pharmacist consecutively numbered the bottles according to the randomization sequence to conceal allocation of the next participant in the sequence |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Once eligibility of participants was confirmed, the investigator used bottles of the study drug consecutively to mask every patient and families, medical staff, and investigators from treatment allocation. All centres received batches of 10 identical bottles, 5 of which contained a solution of the study drug and 5 of which contained a placebo solution. The solutions had identical colour, smell, taste, and viscosity |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | This was a double‐blind study |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 5 participants were withdrawn from the study by their families (1 on rivastigmine and 4 on placebo), leading to a modified intention‐to‐treat analysis of 54 participants on rivastigmine and 50 on placebo. Data were censored for 16 participants who died and for 19 participants who were discharged from hospital while still delirious |
| Selective reporting (reporting bias) | Low risk | All outcomes reported in methods were included in results. Data presented for all participants were included in the analysis |
| Other bias | Low risk | All included participants received usual care, which included frequent orientation, physical therapy, and exercise. The treating physician could adjust treatment with haloperidol or a benzodiazepine, similar to usual care. In case of persistent severe agitation, rescue haloperidol was recommended ZonMw and the Netherlands Brain Foundation did not contribute to study design, data collection, data analysis, data interpretation, writing of the report, or the decision to submit for publication. Novartis had no role in the decision to conduct the study or to stop it early; nor in study design, data collection, data analysis, data interpretation, writing of the report, or the decision to submit for publication |
CAM‐ICU: Confusion Assessment Method for the ICU.
DI: Delirium Index.
D5W: 5% dextrose in water.
ICDSC: Intensive Care Delirium Screening Checklist.
ICU: intensive care unit.
IQR: interquartile range.
MAAS: Motor Activity Assessment Scale.
QTc: measure of time between start of the Q wave and end of the T wave in the heart's electrical cycle corrected for heart rate.
RASS: Richmond Agitation‐Sedation Scale.
RCT: randomized controlled trial.
REB: research ethics board.
SAILS: Statins for Acutely Injured Lungs from Sepsis trial.
SAS: Riker Sedation‐Agitation Scale.
SOFA: Sequential Organ Failure Assessment.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Eremenko 2014 | This study is not a randomized controlled trial. Participants in the intervention group received dexmedetomidine, some exclusively; others also received haloperidol and midazolam. Patients in the control group received haloperidol intramuscularly and intravenously, separately and in combination with benzodiazepines |
| Khan 2019 | The experimental intervention (vs usual care) included a multi‐component pharmacological management of delirium bundle, consisting of reducing exposure to 20 definite anticholinergic medications and benzodiazepines and prescribing low‐dose haloperidol. The usual care group could also receive haloperidol. A full study protocol was published (Campbell 2011) |
| Mailhot 2014 | The intervention had no pharmacological component. The experimental intervention included mentoring family members about delirium management behaviours and offering support for their implementation |
| Pandharipande 2007 | The purpose of this study was to examine the sedative effects of 2 different drugs. Although delirium was a reported secondary outcome for this trial, the focus of the trial was not delirium treatment |
| Riker 2009 | The purpose of this study was to examine efficacy and safety of prolonged sedation with dexmedetomidine vs midazolam for mechanically ventilated patients. Although delirium was a reported secondary outcome for this trial, the focus of the trial was not delirium treatment |
| Tagarakis 2012 | This study did not use a validated delirium screening tool. For detection of delirium, study authors used a 4‐point scale (0 ‐ normal; 1 ‐ patient with restlessness and mild confusion but co‐operative; 2 ‐ patient disoriented but co‐operative, memory gaps; 3 ‐ patient disoriented and unco‐operative with augmented mobility that could put him in danger; 4 ‐ patient totally disoriented, violent, and aggressive, presence of hallucinations) |
| Waszynski 2018 | This study evaluated delirious participants, but no study drug was administered to either group |
Characteristics of studies awaiting assessment [ordered by study ID]
Emerson 2014.
| Methods | Randomized trial comparing the effectiveness of a delirium management team vs standard of care in the treatment of delirium Study took place in 3 medical‐surgical ICUs in the United States |
| Participants | Adult (> 18 years) ICU patients diagnosed with delirium via the CAM‐ICU |
| Interventions | Participants received standard delirium care or management via a team consisting of a physician, a clinical pharmacist, and a registered nurse delirium co‐ordinator. Participants in the intervention group were assessed daily over the course of their ICU stay for non‐pharmacological and pharmacological interventions. Per hospital initiative, a delirium prevention bundle and early mobilization administered by exercise physiologists were provided to all patients in the study |
| Outcomes |
Primary
Secondary
|
| Notes | Study status unknown. Study was published in abstract form under the title "Impact of a collaborative multidisciplinary team on ICU delirium" (Emerson 2014). Several attempts to contact the principal study investigator (kemerson1@stlukeshealth.org) were unsuccessful |
ISRCTN33122761.
| Methods | Randomized trial comparing physostigmine salicylate vs placebo in the treatment of delirium Single centre in Germany |
| Participants | Adult (18 to < 90 years) ICU patients who had undergone elective aortocoronary bypass under mild hypothermia (34°C) and were diagnosed with delirium via the CAM‐ICU |
| Interventions | Participants received physostigmine 0.03 mg per kg body weight administered intravenously or matched placebo |
| Outcomes |
Primary
Secondary
|
| Notes | Study is stated as complete but does not appear to be published Study started in December 2008 and was registered under the name "Efficacy and tolerability of physostigmine salicylate for treatment of post‐operative delirium after aortocoronary‐bypass operation (ACVB): a prospective, double‐blind, placebo‐controlled, 2 parallel‐groups, phase III study" Sponsor: Dr Franz Köhler Chemie GmbH (Germany) Study registered: at isrctn.com under ISRCTN33122761 Several attempts to contact the principal study investigator were unsuccessful (Markus.Verch@med.uni‐heidelberg.de) |
NCT00429676.
| Methods | Randomized trial comparing standard sedation to standard sedation with add‐on haloperidol for duration of mechanical ventilation in patients with delirium Single ICU in the USA |
| Participants | Adult (> 18 years) patients requiring mechanical ventilation within 24 hours of ICU admission and diagnosed with delirium via the CAM‐ICU |
| Interventions | Participants received a standard of care sedation protocol with or without add‐on haloperidol. Haloperidol dose was administered via a titration protocol guided by nursing assessment of delirium using the CAM‐ICU |
| Outcomes |
Primary
Secondary
|
| Notes | Study is complete but unpublished Study was started in December 2005 and was registered under the name "A randomised prospective pilot study of haloperidol in addition to standard sedation in mechanically ventilated patients with delirium" Sponsor: University of Colorado, Denver Study registered: clinicaltrials.gov under NCT00429676 Principal investigator was contacted and was not able to share data for inclusion in this review |
NCT02366299.
| Methods | Randomized trial comparing effects of dexmedetomidine and propofol on delirium and neuroinflammation in patients with systemic inflammatory response syndrome (SIRS) No study location details provided |
| Participants | Adult (18 to < 80 years) participants diagnosed with delirium |
| Interventions | Participants received an infusion of dexmedetomidine or propofol |
| Outcomes |
Primary
Secondary
|
| Notes | Study status unknown. Study registered in February 2015 and listed as not yet recruiting. Study status have not been updated since that time Sponsor: Moscow Regional Research and Clinical Institute Moniki n.a. M.F. Vladimirskiy Study registered: at clinicaltrials.gov under NCT02366299 Unable to contact principal investigator |
Peters 2015.
| Methods | Randomized trial comparing intranasal insulin aspart vs placebo in the treatment of delirium Single‐centre study in the USA |
| Participants | Adult (> 18 years) participants diagnosed with delirium via the CAM‐ICU |
| Interventions | Participants received a single intranasal dose of 40 units insulin aspart or 0.4 mL normal saline at the time of delirium diagnosis. Delirium was reassessed 15 minutes later and daily until discharge |
| Outcomes |
Primary
Secondary
|
| Notes | Study status unknown. Study was published in abstract form under the title "Therapeutic effects of intranasal insulin aspart on cognitive function in postoperative delirium" (Peters 2015). Several attempts to contact the principal study investigator (l‐peters@onu.edu) were unsuccessful |
Schoeffler 2012.
| Methods | Randomized trial comparing clonidine vs placebo in the treatment of delirium Single centre in the USA |
| Participants | Adult (> 18 years) trauma patients admitted to the ICU > 24 hours, meeting criteria for extubation, declared stable from a neurological, respiratory, and cardiovascular standpoint to receive clonidine, and diagnosed with delirium via the CAM‐ICU |
| Interventions | Participants received an oral loading dose of 0.3 mg clonidine or matched placebo, along with a transdermal clonidine patch of the same dose with patch overlay (patch overlay alone in placebo group). 12 hours later, a final dose of 0.3 mg clonidine or matched placebo was given |
| Outcomes |
Primary
Secondary
|
| Notes | Study is stated as terminated in June 2017 due to difficulty recruiting participants . Study was started in May 2010 and was registered under the name "A randomized double blinded placebo controlled trial of transdermal clonidine for adjuvant sedation in ventilated trauma patients experiencing delirium" Interim study results published in abstract form (Schoeffler 2012) Sponsor: Memorial Health University Medical Center (Savannah, Georgia) Study registered: at clinicaltrials.gov under NCT01139996 Principal investigator was contacted and was not able to share data for inclusion in this review (schoeme1@memorialhealth.com) |
CAM‐ICU: Confusion Assessment Method for the ICU.
ICU: intensive care unit.
RASS: Richmond Agitation‐Sedation Scale.
Characteristics of ongoing studies [ordered by study ID]
IRCT20121231011956N10.
| Trial name or title | Comparison of the effectiveness of haloperidol and quetiapine for delirium in the emergency department and intensive care unit |
| Methods | Single‐site, double‐blind, randomized study taking place in Iran. Target enrolment: 100 participants |
| Participants | Adult (> 18 years) patients admitted to the Emergency Department or ICU and diagnosed with delirium via DSM criteria |
| Interventions | Participants randomized to receive haloperidol (5 mg IM daily) or quetiapine (25 mg PO daily) |
| Outcomes |
Primary
Secondary
|
| Starting date | March 2018 |
| Contact information | Morteza Talebi Doluee (talebidm@mums.ac.ir) |
| Notes | Study recruitment is complete Funding source: Mashhad University of Medical Sciences Sponsor: Mashhad University of Medical Sciences Study registered: at https://en.irct.ir/trial/29718 |
IRCT20180911040998N1.
| Trial name or title | Comparison of the effect of quetiapine and haloperidol on the treatment of delirium in ICU |
| Methods | Single‐site, double‐blind, randomized study taking place in Iran. Target enrolment: 60 participants |
| Participants | Adult (> 18 years) ICU patients diagnosed with delirium via DSM criteria |
| Interventions | Participants randomized to receive injected haloperidol (2.5 mg/d) or quetiapine (25 mg twice daily) |
| Outcomes |
Primary
Secondary
|
| Starting date | February 2018 |
| Contact information | Alireza Kamali (alikamaliir@yahoo.com) |
| Notes | Study is currently recruiting Funding source: Arak University of Medical Sciences Sponsor: Arak University of Medical Sciences Study registered: at https://en.irct.ir/trial/33804 |
NCT00351299.
| Trial name or title | Study of dexmedetomidine as an effective sedative to treat acute ICU delirium |
| Methods | Single‐site, double‐blind, randomized study taking place in the United States. Target enrolment: 53 participants |
| Participants | Adult (≥ 18 years) ICU patients diagnosed with delirium via the CAM‐ICU |
| Interventions | Participants randomized to receive an infusion of dexmedetomidine (0.3 to 0.7 mcg/kg/h) or standard of care (according to physician preference, no standard drug dosing, typical and atypical antipsychotic use permitted) |
| Outcomes |
Primary
Secondary
|
| Starting date | January 2006 |
| Contact information | Namrata Patil (npatil@partners.org) Gerald Weinhouse (gweinhouse@partners.org) |
| Notes | This study is complete; 5‐ and 7‐year follow‐up data are being collected as of September 2014 (communication with principal investigators) Study sponsor: Brigham and Women's Hospital Study registered: at clinicaltrials.gov under NCT00351299 |
NCT01811459.
| Trial name or title | Randomized trial comparing haloperidol, quetiapine, and placebo in the pharmacological treatment of delirium (Haloquet) |
| Methods | Single‐site, quadruple‐blind, randomized placebo‐controlled study taking place in Canada. Target enrolment: 107 participants |
| Participants | Adult (≥ 18 years) ICU patients diagnosed with delirium by a psychiatrist (DSM criteria) |
| Interventions | Participants randomized to 1 mg intravenous haloperidol and oral placebo, 50 mg oral quetiapine and intravenous placebo, or intravenous and oral placebo As needed 2 mg intravenous doses of haloperidol permitted every 30 minutes to all participants until delirium symptoms resolve. Incremental titration (1 mg haloperidol or 50 mg quetiapine) permitted twice daily if 2 doses of as needed haloperidol were given in the previous 24 hours. Additional rescue doses of 5 mg of intravenous haloperidol were permitted every 30 minutes to all participants if agreed by the treating physician Treatment continued until:
|
| Outcomes |
Primary
Secondary
|
| Starting date | February 2013 |
| Contact information | Nicholas Bergeron (nbergeron@yahoo.com) Marie‐Pierre Leduc (marie‐pierre.leduc.chum@ssss.gouv.qc.ca) |
| Notes | Study recruitment complete and data analysis phase in progress as of December 2017 (communication with principal investigators) Sponsor: Centre Hopitalier de l'Université de Montréal Study registered: at clinicaltrials.gov under NCT01811459 |
NCT02216266.
| Trial name or title | Monocentre, double‐blind, randomised, placebo‐controlled study to evaluate physostigmine for the treatment of delirium in perioperative intensive care medicine |
| Methods | Single‐site, triple‐blind, randomized placebo‐controlled study taking place in Germany. Target enrolment: 120 participants |
| Participants | Adult (18 to < 85 years) ICU patients post elective or emergency heart surgery (with or without extracorporeal circulation) diagnosed with delirium via the CAM‐ICU |
| Interventions | Participants randomized to receive physostigmine or placebo, administered intravenously at a dose of 24 mg + 25 minutes at 0.04 mg/kg |
| Outcomes |
Primary
Secondary
|
| Starting date | April 2014 |
| Contact information | Bertram Scheller (bertram.scheller@kgu.de) |
| Notes | Study status unknown, but last updated as recruiting on 10 March 2017 Study sponsor: PD Dr Bertram Scheller Study registered: at clinicaltrials.gov under NCT02216266 |
NCT02343575.
| Trial name or title | Valproic acid for treatment of hyperactive or mixed delirium in ICU |
| Methods | Single‐site, quadruple‐blind, randomized placebo‐controlled study taking place in the United States. Target enrolment: 30 participants |
| Participants | Adult (≥ 18 years) ICU patients diagnosed with hyperactive or mixed delirium via the CAM‐ICU |
| Interventions | Participants randomized to receive valproic acid or matched placebo, started at 500 mg twice daily (oral or via nasogastric tube) If additional symptom control needed, increases permitted every 24 hours
All participants permitted as needed rescue haloperidol 2 to 5 mg given intravenously every 4 hours |
| Outcomes |
Primary
Secondary
|
| Starting date | January 2015 |
| Contact information | Yelizaveta Sher, Stanford University Jose R Maldonado, Stanford University |
| Notes | Study is currently suspended due to loss of research staff Study sponsor: Stanford University Study registered: at clinicaltrials.gov under NCT02343575 |
NCT02807467.
| Trial name or title | Comparison of propofol and dexmedetomidine to treat hyperactive and mixed ICU delirium: the Basel ProDex randomised trial |
| Methods | Single‐site, open‐label, randomized study taking place in Switzerland. Target enrolment: 318 participants |
| Participants | Adult (≥ 18 years) ICU patients diagnosed with hyperactive or mixed delirium via ICDSC |
| Interventions | Participants randomized to receive a continuous infusion of dexmedetomidine (200 µg/2 mL) or propofol (1% 1 g/100 mL) between the hours of 20:00 and 06:00. Rescue haloperidol (administered intravenously) used for daytime symptoms of delirium. The dexmedetomidine or propofol infusion was repeated nightly until full resolution of delirium |
| Outcomes |
Primary
Secondary
|
| Starting date | January 2017 |
| Contact information | Alexa Hollinger (alexa.hollinger@usb.ch) Martin Siegemund (martin.siegemund@usb.ch) |
| Notes | Study is currently recruiting Study sponsor: University Hospital, Basel, Switzerland Full protocol published and study registered at clinicaltrials.gov under NCT02807467 (Hollinger 2017) |
NCT03317067.
| Trial name or title | Effects of dexmedetomidine on delirium duration of non‐intubated ICU patients (4D trial) |
| Methods | Multi‐site, double‐blind, randomized, placebo‐controlled trials taking place in France. Target enrolment: 300 participants |
| Participants | Adult (> 18 years), non‐intubated ICU patients diagnosed with delirium (via CAM‐ICU and RASS) |
| Interventions | Participants randomized to receive dexmedetomidine or placebo (NaCL 0.9%) via continuous infusion |
| Outcomes |
Primary
Secondary
|
| Starting date | December 2017 |
| Contact information | Patrick Lacarin (placarin@chu‐clermontferrand.fr) |
| Notes | Study is currently recruiting Study sponsor: University Hospital, Clermont‐Ferrand Full protocol published and study registered at clinicaltrials.gov under NCT03317067 (Louis 2018) |
NCT03392376.
| Trial name or title | Agents intervening against delirium in intensive care unit (AID‐ICU) |
| Methods | Multi‐site (23), quadruple‐blind, randomized placebo‐controlled trial taking place in Denmark, Finland, Germany, Italy, Norway, and Spain. Target enrolment: 1000 patients |
| Participants | Acutely admitted adult (> 18 years) patients diagnosed with delirium via the CAM‐ICU or ICDSC |
| Interventions | Participants randomized to receive haloperidol (2.5 mg IV thrice daily with additional, as needed doses up to a maximum of 20 mg/d) or placebo (0.5 mL IV isotonic saline thrice daily, with additional, as needed doses up to a maximum of 4 mL/d) |
| Outcomes |
Primary
Secondary
|
| Starting date | 12 June 2018 |
| Contact information | Lone Musaeus Poulsen, MD (lmp@regionsjaelland.dk) Nina Christine Andersen‐Ranberg, MD (ncan@regionsjaelland.dk) |
| Notes | Subset of sites currently recruiting Study sponsor: Zealand University Hospital Study registered: at https://clinicaltrials.gov/ct2/show/NCT03392376 |
NCT03628391.
| Trial name or title | Efficacy of halopeRIdol to decrease the burden of Delirium In adult Critically ill patiEnts: a prospective randomized multi‐center double‐blind placebo‐controlled clinical trial (EuRIDICE) |
| Methods | Multi‐site (6), quadruple‐blind, randomized placebo‐controlled trial taking place in the Netherlands. Target enrolment: 742 participants |
| Participants | Adult (≥ 18 years) participants admitted to 1 of 6 participating ICUs and diagnosed with delirium by the CAM‐ICU or ICDSC ≥ 4 |
| Interventions | Participants randomized to receive haloperidol, starting with 2.5 mg IV q8h and titrated to a maximum of 5 mg IV q8h, or placebo |
| Outcomes |
Primary
Secondary
|
| Starting date | February 2018 |
| Contact information | Mathieu van der Jagt (m.vanderjagt@erasmusmc.nl) |
| Notes | Study is currently recruiting Study sponsor: Erasmus Medical Center Rotterdam Study funded by: ZonMw Study registered: at https://www.clinicaltrialsregister.eu/ctr‐search/trial/2017‐003115‐20/NL |
APACHE: Acute Physiology and Chronic Healh Evaluation.
CAM‐ICU: Confusion Assessment Method for the ICU.
DRS‐R‐98: Delirium Rating Scale ‐ Revised ‐ 98.
DSM: Diagnostic and Statistical Manual.
EEG: electroencephalography.
EQ‐5D‐5L: EuroQoL Group Quality of Life Based on 5 Dimensions ‐ 5‐Level Scale.
ICDSC: Intensive Care Delirium Screening Checklist.
ICU: intensive care unit.
QTc: measure of time between start of the Q wave and end of the T wave in the heart's electrical cycle corrected for heart rate.
RASS: Richmond Agitation‐Sedation Scale.
Differences between protocol and review
In our protocol (Burry 2015), we planned the primary outcome to be duration of delirium, defined as the time from which it wasfirst identified to when it was first resolved (i.e. screened negative as defined by study authors (e.g. first negative screen, two consecutive screenings)), measured in days, and our secondary outcome to be the total duration of delirium, measured in days. There was far more variability in the definition of the outcome used than we had anticipated. Only two trials reported on the duration of delirium's first episode, and the remaining trials reported days with delirium, time in delirium, or total duration of delirium; most did not report when delirium was identified or how trial authors defined resolution of delirium. We therefore chose to report the total duration of delirium as our primary outcome and to pool the variable definitions. We added the outcome number of days in coma, as this outcome was reported in four trials, and we believed it important to include it in this review, as it is a newer outcome that is likely to be included in subsequent studies.
We chose not to report mortality in our 'Summary of findings' tables, as this was reported at various time points and settings, and NMA was judged by the research team to be inappropriate to pursue. Also, mortality is more likely the consequence of the same causes that led patients to become critically ill, rather than the intervention for delirium. In its place, we chose to report the most commonly reported adverse drug effect ‐ QTc prolongation. We also chose to report "days with coma" in place of "delirium‐free and coma‐free days", given that the RoM analysis for "days with coma" is relatively more reliable and is less impacted by the different time windows of measurement.
Estimates from NMAs are included in the 'Summary of findings' tables, in addition to estimates from pairwise meta‐analyses. The mean difference on the log scale from pairwise meta‐analyses was exponentiated and interpreted as RoM, which we presented alongside estimates from the NMAs, to provide estimates both from direct comparisons (i.e. pairwise meta‐analyses) and from mixed comparisons (i.e. NMAs). In the case of no direct evidence available between an intervention and placebo, we presented only the estimate of indirect comparison from NMA. SUCRA values, mean rankings, and Pr(‘best’) values were provided as additional measures of treatment effect, which are commonly of interest to readers.
Contributions of authors
Neill KJ Adhikari (NA), Lisa Burry (LB), Wei Cheng (WC), Ingrid Egerod (IE), E. Wes Ely (EWE), Dean A Fergusson (DF), Brian Hutton (BH), Sangeeta Mehta (SM), Louise Rose (LR), David R Williamson (DW).
Conceiving the review: LB, LR.
Designing the review: LB, LR, EWE, BH.
Co‐ordinating the review: LB.
Undertaking manual searches: LB.
Screening search results: LB, LR.
Organizing retrieval of papers: LB.
Screening retrieved papers against inclusion criteria: LB, LR.
Appraising quality of papers: SM, DW, NA, IE, LB.
Abstracting data from papers: SM, DW, NA, IE.
Writing to authors of papers for additional information: LB.
Providing additional data about papers: LB.
Obtaining and screening data on unpublished studies: LB.
Managing data for the review: WC, LB, BH.
Analysing Review Manager statistical data: WC, BH, LB.
Performing other statistical analysis not using RevMan: WC, BH.
Making statistical inferences: BH, WC, DF.
Interpreting data: all.
Writing the protocol and review: LB, BH, WC, EWE, LR.
Serving as guarantor for the review (one author): LB.
Taking responsibility for reading and checking the review before submission: LB.
Sources of support
Internal sources
-
Department of Pharmacy, Mount Sinai Hospital, Toronto, Ontario, Canada.
Internal funding for paper supplies and computer software.
External sources
-
Canadian Institutes of Health Research (NRF: 144048), Canada.
Academic grant funding. Granting agency had no involvement in design or completion of the review
Declarations of interest
None to declare: Lisa Burry, Neill KJ Adhikari, Wei Cheng, Ingrid Egerod, Dean A Fergusson, Brian Hutton, Sangeeta Mehta, Louise Rose, and David R Williamson.
To declare:
Dr Hutton has previously received honoraria from Cornerstone Research Group for providing methodological advice related to systematic reviews and meta‐analyses. This company does not market any products relevant to this review or therapeutic area.
Dr Ely is an author on four RCTs that met our inclusion criteria (i.e. Girard 2010a; Girard 2018; Page 2013; Page 2017). He was not involved in data extraction or risk of bias assessment for any of the RCTs. He is also supported by NIH funding, which is paid directly to Vanderbilt University. Dr Ely had received funds as honoraria for evidence‐based teaching activities from Orion, Hospira, and Abbott Laboratories education divisions (2015‐2017). At this time, these companies do not produce or market drugs with a specific indication for the treatment of delirium. They do market the sedative drug dexmedetomidine, which is included in this review. Dr Ely is not an author on the dexmedetomidine study included in this review. Dr Ely is currently studying as a co‐investigator in an NIH‐sponsored clinical trial on the off‐label use of delirium treatment.
Edited (no change to conclusions)
References
References to studies included in this review
Al‐Qadheeb 2016 {published data only}
- Al‐Qadheeb NS, Skrobik Y, Schumaker G, Pacheco MN, Roberts RJ, Ruthazer RR, et al. Preventing ICU subsyndromal delirium conversion to delirium with low‐dose IV haloperidol: a double‐blind, placebo‐controlled pilot study. Critical Care Medicine 2016;44(3):583‐91. [PUBMED: 26540397] [DOI] [PMC free article] [PubMed] [Google Scholar]
Atalan 2013 {published data only}
- Atalan N, Sevim ME, Akgun S, Fazligullari O, Basaran C. Morphine is a reasonable alternative to haloperidol in the treatment of postoperative hyperactive‐type delirium after cardiac surgery. Journal of Cardiothoracic and Vascular Anesthesia 2013;27(5):933‐8. [PUBMED: 23791495] [DOI] [PubMed] [Google Scholar]
Bakri 2015 {published data only}
- Bakri MH, Ismail EA, Ibrahim A. Comparison of dexmedetomidine or ondansetron with haloperidol for treatment of postoperative delirium in trauma patients admitted to intensive care unit: randomized controlled trial. Anaesthesia, Pain and Intensive Care 2015;19(2):118‐23. [EMBASE: 606162167] [Google Scholar]
Devlin 2010 {published data only}
- Devlin JW, Robert RJ, Fong JJ, Skrobik Y, Riker RR, Hill NS, et al. Efficacy and safety of quetiapine in critically ill patients with delirium: a prospective, multicenter, randomized, double‐blind, placebo‐controlled pilot study. Critical Care Medicine 2010;38(2):419‐27. [PUBMED: 19915454 ] [DOI] [PubMed] [Google Scholar]
Girard 2010a {published data only}
- Girard TD, Pandharipande PP, Carson SS, Schmidt GA, Wright PE, Canonico AE, et al. Feasibility, efficacy, and safety of antipsychotics for intensive care unit delirium: the MIND randomized, placebo‐controlled trial. Critical Care Medicine 2010;38(2):428‐37. [PUBMED: 20095068] [DOI] [PMC free article] [PubMed] [Google Scholar]
Girard 2018 {published data only}
- Girard TD, Exline MC, Carson SS, Hough CL, Rock P, Gong MN, MIND‐USA Investigators. Haloperidol and ziprasidone for treatment of delirium in critical illness. New England Journal of Medicine 2018;379(26):2506‐16. [PUBMED: 30346242] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hakim 2012 {published data only}
- Hakim SM, Othman AI, Naoum DO. Early treatment with risperidone for subsyndromal delirium after on‐pump cardiac surgery in the elderly: a randomized trial. Anesthesiology 2012;116(5):987‐97. [PUBMED: 22436797 ] [DOI] [PubMed] [Google Scholar]
Needham 2016 {published and unpublished data}
- Needham DM, Colantuoni E, Dinglas VD, Hough CL, Wozniak AW, Jackson JC, et al. Rosuvastatin versus placebo for delirium in intensive care and subsequent cognitive impairment in patients with sepsis‐associated acute respiratory distress syndrome: an ancillary study to a randomised controlled trial. Lancet Respiratory Medicine 2016;4(3):203‐12. [PUBMED: 26832963 ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Page 2013 {published data only}
- Page VJ, Ely EW, Gates S, Zhao XB, Alce T, Shintani A, et al. Effect of intravenous haloperidol on the duration of delirium and coma in critically ill patients (Hope‐ICU): a randomised, double‐blind, placebo‐controlled trial. Lancet Respiratory Medicine 2013;1(7):515‐23. [PUBMED: 24461612 ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Page 2017 {published data only}
- Page VJ, Casarin A, Ely EW, Zhao XB, McDowell C, Murphy L, et al. Evaluation of early administration of simvastatin in the prevention and treatment of delirium in critically ill patients undergoing mechanical ventilation (MoDUS): a randomised, double‐blind, placebo‐controlled trial. Lancet Respiratory Medicine 2017;5(9):727‐37. [PUBMED: 28734823] [DOI] [PubMed] [Google Scholar]
Reade 2009 {published data only}
- Reade MC, O'Sullivan K, Bates S, Goldsmith D, Ainslie WR, Bellomo R. Dexmedetomidine vs. haloperidol in delirious, agitated, intubated patients: a randomised open‐label trial. Critical Care 2009;13(3):R75. [PUBMED: 19454032 ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Reade 2016 {published data only}
- Reade MC, Eastwood GM, Bellomo R, Bailey M, Bersten A, Cheung B, et al. Effect of dexmedetomidine added to standard care on ventilator‐free time in patients with agitated delirium: a randomized clinical trial. JAMA 2016;315(14):1460‐8. [PUBMED: 26975647 ] [DOI] [PubMed] [Google Scholar]
Skrobik 2004 {published data only}
- Skrobik Y, Bergeron N, Dumont M, Gottfried SB. Olanzapine vs haloperidol: treating delirium in a critical care setting. Intensive Care Medicine 2004;30(3):444‐9. [PUBMED: 14685663] [DOI] [PubMed] [Google Scholar]
van Eijk 2010 {published data only}
- Eijk MM, Roes KC, Honing ML, Kuiper MA, Karakus A, Jagt M, et al. Effect of rivastigmine as an adjunct to usual care with haloperidol on duration of delirium and mortality in critically ill patients: a multicentre, double‐blind, placebo‐controlled randomised trial. Lancet 2010;376(9755):1829‐37. [PUBMED: 21056464] [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Eremenko 2014 {published data only}
- Eremenko AA, Chernova EV. Treatment of delirium in the early postoperative period after cardiac surgery. Anesteziologiia i Reanimatologiia 2014;May‐Jun(3):30‐4. [PUBMED: 25306681] [PubMed] [Google Scholar]
Khan 2019 {published data only}
- Khan BA, Perkins AJ, Campbell NL, Gao S, Farber MO, Wang S, et al. Pharmacological management of delirium in the intensive care unit: a randomized pragmatic clinical trial. Journal of the American Geriatric Society 2019;E‐pub ahead of print. [PUBMED: 30681720] [DOI] [PMC free article] [PubMed] [Google Scholar]
Mailhot 2014 {published data only}
- Mailhot T, Cossette S, Denault AY, Lamarche Y, Cote M‐C, Carbonneau M‐H, et al. Nursing intervention involving family caregiver to improve the management of post‐cardiac surgery delirium: results from a randomized pilot study. Canadian Journal of Cardiology. 2014; Vol. 30, issue 10:Suppl S353.
Pandharipande 2007 {published data only}
- Pandharipande PP, Pun BT, Herr DL, Maze M, Girard TD, Miller RR, et al. Effect of sedation with dexmedetomidine vs lorazepam on acute brain dysfunction in mechanically ventilated patients: the MENDS randomized controlled trial. JAMA 2007;298(22):2644‐53. [PUBMED: 18073360] [DOI] [PubMed] [Google Scholar]
Riker 2009 {published data only}
- Riker RR, Shehabi Y, Bokesch PM, Ceraso D, Wisemandle W, Koura F, et al. Dexmedetomidine vs midazolam for sedation of critically ill patients: a randomized trial. JAMA 2009;301(5):489‐99. [DOI: 10.1001/jama.2009.56] [DOI] [PubMed] [Google Scholar]
Tagarakis 2012 {published data only}
- Tagarakis GI, Voucharas C, Tsolaki F, Daskalopoulos ME, Papaliagkas V, Parisis C, et al. Ondasetron versus haloperidol for the treatment of postcardiotomy delirium: a prospective, randomized, double‐blinded study. Journal of Cardiothoracic Surgery 2012;21(7):25. [PUBMED: 22436170] [DOI] [PMC free article] [PubMed] [Google Scholar]
Waszynski 2018 {published data only}
- Waszynski CM, Milner KA, Staff I, Molony SL. Using simulated family presence to decrease agitation in older hospitalized delirious patients: a randomized controlled trial. International Journal of Nursing Studies 2018;77:154‐61. [PUBMED: 29100197] [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
Emerson 2014 {published data only}
- Emerson K, Hu B, Smith C, Kopas L, Wang S, Grami P, et al. Impact of a collaborative multidisciplinary team on ICU delirium. Critical Care Medicine. 2014; Vol. 42, issue 12:A1501‐2. [00003246‐201412001‐00556]
ISRCTN33122761 {published data only}
- ISRCTN33122761. Efficacy and tolerability of physostigmine salicylate for treatment of post‐operative delirium after aortocoronary‐bypass operation (ACVB): a prospective, double‐blind, placebo‐controlled, two parallel‐groups, phase III study. isrctn.com/ISRCTN33122761 (first received 17 October 2008).
NCT00429676 {published data only}
- NCT00429676. Pilot study of haloperidol to treat critical illness delirium [A randomized prospective pilot study of haloperidol in addition to standard sedation in mechanically ventilated patients with delirium]. clinicaltrials.gov/ct2/show/NCT00429676 (first received 1 February 2007).
NCT02366299 {published data only}
- NCT02366299. Comparison of dexmedetomidine and propofol on the delirium and neuroinflammation in patients with SIRS [Comparison of sedative effects of sevoflurane and dexmedetomidine on the clinical course of delirium and neuroinflammation in patients with SIRS]. https://clinicaltrials.gov/ct2/show/NCT02366299 (first received 19 February 2015).
Peters 2015 {published data only}
- Peters L, Melashenko R, Webster M, Kauflin M, Hithe K, Wolters N, et al. Therapeutic effects of intranasal insulin aspart on cognitive function in postoperative delirium. Journal of Pharmacy Practice. 2015; Vol. 28:315‐6.
Schoeffler 2012 {published data only}
- Schoeffler M. A randomized double blinded placebo controlled trial of transdermal clonidine for adjuvant sedation in ventilated trauma patients experiencing delirium. Critical Care Medicine. 2012; Vol. 40:286. [DOI: 10.1097/01.ccm.0000425343.95028.64] [DOI]
References to ongoing studies
IRCT20121231011956N10 {published data only}
- IRCT20121231011956N10. Comparison of the effectiveness of haloperidol and quetiapine for delirium in the emergency department and intensive care unit [Effectiveness of haloperidol and quetiapine in delirium]. https://en.irct.ir/trial/29718 (first received 21 April 2018).
IRCT20180911040998N1 {published data only}
- IRCT20180911040998N1. Comparison of the effect of quetiapine and haloperidol on the treatment of delirium in ICU. https://en.irct.ir/trial/33804 (first received 31 October 2018).
NCT00351299 {published data only}
- NCT00351299. Randomized controlled trial of dexmedetomidine for the treatment of intensive care unit (ICU) delirium [Study of dexmedetomidine as an effective sedative to treat acute ICU delirium]. clinicaltrials.gov/ct2/show/NCT00351299 (first received 12 July 2006).
NCT01811459 {published data only}
- NCT01811459. Trial comparing haloperidol, quetiapine and placebo in the pharmacological treatment of delirium (Haloquet) [Randomized controlled trial comparing haloperidol, quetiapine and placebo in the pharmacological treatment of delirium: the Haloquet trial]. clinicaltrials.gov/ct2/show/NCT01811459 (first received 14 March 2013).
NCT02216266 {published data only}
- NCT02216266. Evaluation if physostigmine reduces symptoms in patients who has developed a delirium in intensive care after a surgery [Monocenter, double blind, randomised, placebo controlled study to evaluate physostigmine for the treatment of delirium in perioperative intensive care medicine]. clinicaltrials.gov/ct2/show/NCT02216266 (first received 13 August 2014).
NCT02343575 {published data only}
- NCT02343575. Valproic acid for treatment of hyperactive or mixed delirium in ICU. clinicaltrials.gov/ct2/show/NCT02343575 (first received 22 January 2015).
NCT02807467 {published data only}
- NCT02807467. Influence of dexmedetomidine or propofol on ICU delirium [Comparison of propofol and dexmedetomidine to treat hyperactive and mixed ICU delirium ‐ the Basel ProDex randomized trial]. clinicaltrials.gov/ct2/show/NCT02807467 (first received 21 June 2016).
NCT03317067 {published data only}
- NCT03317067. Effects of dexmedetomidine on delirium duration of non‐intubated ICU patients (4D trial). clinicaltrials.gov/ct2/show/NCT03317067 (first received 23 October 2017).
NCT03392376 {published data only}
- NCT03392376. Agents Intervening against delirium in intensive care unit (AID‐ICU) [Agents intervening against delirium in intensive care unit (AID‐ICU): a randomized, blinded, placebo‐controlled trial]. https://clinicaltrials.gov/ct2/show/NCT03392376 (first received 8 January 2018).
NCT03628391 {published data only}
- NCT03628391. Haloperidol for delirium in adult critically ill patients (EuRIDICE) [Efficacy of haloperidol to decrease the burden of delirium in adult critically ill patients: a prospective randomised multi‐center double‐blind placebo‐controlled clinical trial (EuRIDICE)]. https://clinicaltrials.gov/ct2/show/NCT03628391 (first received 14 August 2018). [DOI] [PMC free article] [PubMed]
Additional references
Al‐Qadheeb 2014
- Al‐Qadheeb NS, Balk EM, Fraser GL, Skrobik Y, Riker RR, Kress JP, et al. Randomized ICU trials do not demonstrate an association between interventions that reduce delirium duration and short‐term mortality: a systematic review and meta‐analysis. Critical Care Medicine 2014;42(6):1442‐54. [PUBMED: 24557420 ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Andrew 2006
- Andrew MK, Freter SH, Rockwood K. Incomplete functional recovery after delirium in elderly people: a prospective cohort study. BMC Geriatrics 2005;5:5. [DOI: 10.1186/1471-2318-5-5; PUBMED: 15774005 ] [DOI] [PMC free article] [PubMed] [Google Scholar]
APA 2013
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th Edition. Arlington, VA, USA: American Psychiatric Association, 2013. [Google Scholar]
Barr 2013
- Barr J, Fraser GL, Puntillo K, Ely EW, Gelinas C, Dasta JF, et al. Clinical practice guidelines for the management of pain, agitation, and delirium in adult patients in the intensive care unit. Critical Care Medicine 2013;41(1):263‐306. [PUBMED: 23269131] [DOI] [PubMed] [Google Scholar]
Bell 2007
- Bell CM, Fischer HD, Rochon PA, Zagorski B, Sykora K, Wodchis WP, et al. Initiation of benzodiazepines in the elderly after hospitalization. Journal of General Internal Medicine 2007;22(7):1024‐9. [PUBMED: 17453266 ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bergeron 2001
- Bergeron N, Dubois MJ, Dumont M, Dial S, Skrobik Y. Intensive care delirium screening checklist: evaluation of a new screening tool. Intensive Care Medicine 2001;27(5):859‐64. [PUBMED: 11430542] [DOI] [PubMed] [Google Scholar]
Black 2011
- Black P, Boore JR, Parahoo K. The effect of nurse‐facilitated family participation in the psychological care of the critically ill patient. Journal of Advanced Nursing 2011;67(5):1091‐101. [PUBMED: 21214624 ] [DOI] [PubMed] [Google Scholar]
Briskman 2010
- Briskman I, Dubinski R, Barak Y. Treating delirium in a general hospital: a descriptive study of prescribing patterns and outcomes. International Psychogeriatrics 2010;22(2):328‐31. [PUBMED: 19785917] [DOI] [PubMed] [Google Scholar]
Brooks 1998
- Brooks SP, Gelman A. Alternative methods for monitoring convergence of iterative simulations. Journal of Computational and Graphical Statistics 1998;7(4):434‐55. [Google Scholar]
Bruera 2009
- Bruera E, Bush SH, Willey J, Paraskevopoulos T, Li Z, Palmer JL, et al. Impact of delirium and recall on the level of distress in patients with advanced cancer and their family caregivers. Cancer 2009;115(9):2004‐12. [PUBMED: 19241420] [DOI] [PMC free article] [PubMed] [Google Scholar]
Burry 2014
- Burry LD, Williamson DR, Perreault MM, Rose L, Cook DJ, Ferguson ND, et al. Analgesic, sedative, antipsychotic, and neuromuscular blocker use in Canadian intensive care units: a prospective, multicentre, observational study. Canadian Journal of Anaesthesia 2014;61(7):619‐30. [PUBMED: 24788564] [DOI] [PubMed] [Google Scholar]
Burry 2017
- Burry LD, Williamson DR, Mehta S, Perreault MM, Mantas I, Mallick R, et al. Delirium and exposure to psychoactive medications in critically ill adults: a multi‐centre observational study. Journal of Critical Care 2017;42:268‐74. [PUBMED: 28806561] [DOI] [PubMed] [Google Scholar]
Caldwell 2005
- Caldwell DM, Ades AE, Higgins JPT. Simultaneous comparison of multiple treatments: combining direct and indirect evidence. BMJ 2005;331(7521):897–900. [PUBMED: 16223826 ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Campbell 2011
- Campbell N, Khan B, Farber M, Campbell T, Perkins A, Hui S, et al. Improving delirium care in the intensive care unit: the design of a pragmatic study. Trials 2011;12(1):139. [PUBMED: 21645330] [DOI] [PMC free article] [PubMed] [Google Scholar]
Cerejeira 2011
- Cerejeira J, Batista P, Nogueira V, Firmino H, Vaz‐Serra A, Mukaetova‐Ladinska EB. Low preoperative plasma cholinesterase activity as a risk marker of postoperative delirium in elderly patients. Age and Ageing 2011;40(5):621‐6. [PUBMED: 21576115] [DOI] [PubMed] [Google Scholar]
Chen 2015
- Chen K, Lu Z, Xin YC, Cai Y, Chen Y, Pan SM. Alpha‐2 agonists for long‐term sedation during mechanical ventilation in critically ill patients. Cochrane Database of Systematic Reviews 2015, Issue 1. [DOI: 10.1002/14651858.CD010269.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
de Rooji 2007
- Rooji SE, Munster BC, Korevaar JC, Levi M. Cytokines and acute phase response in delirium. Journal of Psychosomatic Research 2007;62(5):521‐5. [PUBMED: 17467406] [DOI] [PubMed] [Google Scholar]
DeMets 1987
- DeMets DL. Methods for combining randomized clinical trials. Statistics in Medicine 1987;6(3):341‐50. [DOI] [PubMed] [Google Scholar]
DerSimonian 1986
- DerSimonian R, Laird N. Meta‐analysis in clinical trials. Controlled Clinical Trials 1986;7(3):177‐88. [PUBMED: 3802833] [DOI] [PubMed] [Google Scholar]
Devlin 2011
- Devlin JW, Skrobik Y, Riker R, Hinderleider E, Roberts R, Fong J, et al. Impact of quetiapine on resolution of individual delirium symptoms in critically ill patients with delirium: a post‐hoc analysis of a double‐blind, randomized, placebo‐controlled study. Critical Care 2011;15(5):R215. [DOI: 10.1186/cc10450; PUBMED: 21923923 ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Dias 2011a
- Dias S, Welton N, Sutton A, Caldwell D, Lu G, Ades A. NICE DSU Technical Support Document 4: inconsistency in networks of evidence based on randomised controlled trials. http://nicedsu.org.uk/technical‐support‐documents/evidence‐synthesis‐tsd‐series/ May 2011 (last updated April 2014; accessed 30 November 2018). [PubMed]
Dias 2011b
- Dias S, Welton N, Sutton A, Ades A. NICE DSU Technical Support Document 2: a generalised linear modelling framework for pairwise and network meta‐analysis of randomised controlled trials. http://nicedsu.org.uk/technical‐support‐documents/evidence‐synthesis‐tsd‐series/ August 2011 (last updated September 2016; accessed 30 November 2018). [PubMed]
Dias 2013
- Dias S, Sutton A, Welton N, Ades A. Evidence synthesis for decision making 3: heterogeneity‐subgroups, meta‐regression, bias, and bias‐adjustment. Medical Decision Making 2013;33(5):618‐40. [PUBMED: 23804507] [DOI] [PMC free article] [PubMed] [Google Scholar]
Donegan 2013
- Donegan S, Williamson P, D'Alessandro U, Tudur SC. Assessing the key assumptions of network meta‐analysis: a review of methods. Research Synthesis Methods 2013;4(4):291‐323. [PUBMED: 26053945] [DOI] [PubMed] [Google Scholar]
Ebersoldt 2007
- Ebersoldt M, Sharshar T, Annane D. Sepsis‐associated delirium. Intensive Care Medicine 2007;33(6):941‐50. [PUBMED: 17410344] [DOI] [PubMed] [Google Scholar]
Ely 2001a
- Ely EW, Inouye SK, Bernard GR, Gordon S, Francis J, May L, et al. Delirium in mechanically ventilated patients: validity and reliability of the confusion assessment method for the intensive care unit (CAM‐ICU). JAMA 2001;286(21):2703‐10. [PUBMED: 11730446] [DOI] [PubMed] [Google Scholar]
Ely 2001b
- Ely EW, Gautam S, Margolin R, Francis J, May L, Speroff T, et al. The impact of delirium in the intensive care unit on hospital length of stay. Intensive Care Medicine 2001;27:1892‐900. [PUBMED: 11797025 ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ely 2004
- Ely EW, Shintani A, Truman B, Speroff T, Gordon SM, Harrell FE Jr, et al. Delirium as a predictor of mortality in mechanically ventilated patients in the intensive care unit. JAMA 2004;291(14):1753‐62. [PUBMED: 15082703 ] [DOI] [PubMed] [Google Scholar]
Ely 2007
- Ely EW, Girard TD, Shintani AK, Jackson JC, Gordon SM, Thomason JW, et al. Apolipoprotein E4 polymorphism as a genetic predisposition to delirium in critically ill patients. Critical Care Medicine 2007;35(1):112‐7. [PUBMED: 17133176] [DOI] [PubMed] [Google Scholar]
Endnote [Computer program]
- EndNote. Version X6. Carlsbad, CA, USA: Thomson Reuters, 2014.
Flacker 1999
- Flacker JM, Lipsitz LA. Neural mechanisms of delirium: current hypotheses and evolving concepts. Journals of Gerontology. Series A, Biological Sciences and Medical Sciences 1999;54(6):B239‐46. [PUBMED: 10411009] [DOI] [PubMed] [Google Scholar]
Fraser 2013
- Fraser GL, Devlin JW, Worby CP, Alhazzani W, Barr J, Dasta JF, et al. Benzodiazepine versus nonbenzodiazepine‐based sedation for mechanically ventilated, critically ill adults: a systematic review and meta‐analysis of randomized trials. Critical Care Medicine 2013;41(9 Suppl 1):S30‐8. [PUBMED: 23989093 ] [DOI] [PubMed] [Google Scholar]
Gelman 1996
- Gelman A. Inference and monitoring convergence. In: Gilks WR, Richardson S, Spiegelhalter DJ editor(s). Markov Chain Monte Carlo in Practice. London: Chapman & Hall, 1996:131‐43. [Google Scholar]
Gill 2007
- Gill SS, Bronskill SE, Normand SL, Anderson GM, Sykora K, Lam K, et al. Antipsychotic drug use and mortality in older adults with dementia. Annals of Internal Medicine 2007;146(11):775‐86. [PUBMED: 17548409] [DOI] [PubMed] [Google Scholar]
Girard 2010b
- Girard TD, Jackson JC, Pandharipande PP, Pun BT, Thompson JL, Shintani AK, et al. Delirium as a predictor of long‐term cognitive impairment in survivors of critical illness. Critical Care Medicine 2010;38(7):1513‐20. [PUBMED: 20473145] [DOI] [PMC free article] [PubMed] [Google Scholar]
GRADEpro GDT [Computer program]
- McMaster University (developed by Evidnce Prime). GRADEpro GDT. Hamilton (ON): McMaster University (developed by Evidnce Prime), 2015. Accessed April 2018.
Gunther 2008
- Gunther ML, Morandi A, Ely EW. Pathophysiology of delirium in the intensive care unit. Critical Care Clinics 2008;24(1):45‐65. [PUBMED: 18241778] [DOI] [PubMed] [Google Scholar]
Herling 2018
- Herling SF, Greve IE, Vasilevskis EE, Egerod I, Bekker Mortensen C, Moller AM, et al. Interventions for preventing intensive care unit delirium in adults. Cochrane Database of Systematic Reviews 2018, Issue 11. [DOI: 10.1002/14651858.CD009783.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 1996
- Higgins J, Whitehead A. Borrowing strength from external trials in a meta‐analysis. Statistics in Medicine 1996;15(24):2733‐49. [PUBMED: 8981683 ] [DOI] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ 2003;327(7414):557–60. [PUBMED: 12958120 ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2008
- Higgins JP, White IR, Anzures‐Cabrera J. Meta‐analysis of skewed data: combining results reported on log‐transformed or raw scales. Statistics in Medicine 2008;27(29):1072‐92. [PUBMED: 18800342 ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Hipp 2012
- Hipp DM, Ely EW. Pharmacological and non‐pharmacological management of delirium in critically ill patients. Neurotherapeutics 2012;9(1):158‐75. [PUBMED: 22270810] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hollinger 2017
- Hollinger A, Ledergerber K, Felten S, Sutter R, Ruegg S, Gantner L, et al. Comparison of propofol and dexmedetomidine infused overnight to treat hyperactive and mixed ICU delirium: a protocol for the Basel ProDex clinical trial. BMJ Open 2017;7(7):e015783. [PUBMED: 28710219 ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hutton 2015
- Hutton B, Salanti G, Caldwell DM, Chaimani A, Schmid CH, Cameron C, et al. The PRISMA extension statement for reporting of systematic reviews incorporating network meta‐analyses of health care interventions: checklist and explanations. Annals of Internal Medicine 2015;162(11):777‐84. [PUBMED: 26030634] [DOI] [PubMed] [Google Scholar]
IHI 2015
- Institute for Healthcare Improvement. http://www.ihi.org/ (accessed 2 September 2014).
Inouye 2006a
- Inouye SK. Delirium in older persons. New England Journal of Medicine 2006;354(11):1157‐65. [PUBMED: 16540616 ] [DOI] [PubMed] [Google Scholar]
Inouye 2006b
- Inouye SK, Ferrucci L. Elucidating the pathophysiology of delirium and the interrelationship of delirium and dementia. Journals of Gerontology. Series A, Biological Sciences and Medical Sciences 2006;61(12):1277‐80. [PUBMED: 17234820 ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Inouye 2014
- Inouye SK, Westendorp RG, Saczynski JS. Delirium in elderly people. Lancet 2014;383(9920):911‐22. [PUBMED: 23992774] [DOI] [PMC free article] [PubMed] [Google Scholar]
Jackson 2004
- Jackson JC, Gordon SM, Hart RP, Hopkins Ro, Ely EW, et al. The association between delirium and cognitive decline: a review of the empirical literature. Neuropsychology Review 2004;14(2):87‐98. [PUBMED: 15264710] [DOI] [PubMed] [Google Scholar]
Jorm 1994
- Jorm AF. A short form of the Informant Questionnaire on Cognitive Decline in the Elderly (IQCODE): development and cross‐validation. Psychological Medicine 1994;24(1):145‐53. [PUBMED: 8208879] [DOI] [PubMed] [Google Scholar]
Kishi 2016
- Kishi T, Hirota T, Matsunaga S, Iwata N. Antipsychotic medications for the treatment of delirium: a systematic review and meta‐analysis of randomised controlled trials. Journal of Neurology, Neurosurgery, and Psychiatry 2016;87(7):767‐74. [PUBMED: 26341326] [DOI] [PubMed] [Google Scholar]
Lachman 2008
- Lachman ME, Tun PA. Handbook of Cognitive Aging: Interdisciplinary Perspectives. Thousand Oaks, CA: Sage Publications, 2008. [Google Scholar]
Leslie 2008
- Leslie DL, Marcantonio ER, Zhang Y, Leo‐Summers L, Inouye SK. One‐year health care costs associated with delirium in the elderly population. Archives of Internal Medicine 2008;168(1):27‐32. [PUBMED: 18195192] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lin 2004
- Lin SM, Liu CY, Wang CH, Lin HC, Huang CD, Huang PY, et al. The impact of delirium on the survival of mechanically ventilated patients. Critical Care Medicine 2004;32(11):2254‐9. [PUBMED: 15640638] [DOI] [PubMed] [Google Scholar]
Lonergan 2007
- Lonergan E, Britton AM, Luxenberg J, Wyller T. Antipsychotics for delirium. Cochrane Database of Systematic Reviews 2007, Issue 2. [DOI: 10.1002/14651858.CD005594.pub2] [DOI] [PubMed] [Google Scholar]
Louis 2018
- Louis C, Godet T, Chanques G, Bourguignon N, Morand D, Pereira B, et al. Effects of dexmedetomidine on delirium duration of non‐intubated ICU patients (4D trial): study protocol for a randomized trial. Trials 2018;19(1):307. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lu 2006
- Lu G, Ades A. Assessing evidence inconsistency in mixed treatment comparisons. Journal of American Statistical Association 2006;101(474):447‐59. [DOI: 10.1198/016214505000001302] [DOI] [Google Scholar]
Lunn 2000
- Lunn DJ, Thomas A, Best N, Spiegelhalter D. WinBUGS ‐ a Bayesian modelling framework: concepts, structure, and extensibility. Statistics and Computing 2000;10(4):325‐37. [DOI: 10.1023/A:1008929526011] [DOI] [Google Scholar]
MacSweeney 2009
- MacSweeney R, Barber V, Page V, Ely EW, Perkins GD, Young JD, et al. A national survey of the management of delirium in UK intensive care units. Quarterly Journal of Medicine: Monthly Journal of the Association of Physicians 2010;103(4):243‐51. [PUBMED: 20139102 ] [DOI] [PubMed] [Google Scholar]
Maldonado 2009
- Maldonado JR, Wysong A, Starre PJ, Block T, Miller C, Reitz BA, et al. Dexmedetomidine and the reduction of postoperative delirium after cardiac surgery. Psychosomatics 2009;50(3):206‐17. [PUBMED: 19567759 ] [DOI] [PubMed] [Google Scholar]
Milbrant 2004
- Milbrant EB, Deppen S, Harrison PL, Shintani AK, Speroff T, Stiles RA, et al. Costs associated with delirium in mechanically ventilated patients. Critical Care Medicine 2004;32(4):955‐62. [PUBMED: 15071384] [DOI] [PubMed] [Google Scholar]
Moher 2009
- Moher D, Liberati A, Tetzlaff J, Altman D, PRISMA Group. Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. PLoS Medicine 2009;6(7):e1000097. [PUBMED: 19621072] [DOI] [PMC free article] [PubMed] [Google Scholar]
Morita 2004
- Morita T, Hirai K, Sakaguchi Y, Tsuneto S, Shima Y. Family‐perceived distress from delirium‐related symptoms of terminally ill cancer patients. Psychosomatics 2004;45(2):107‐13. [PUBMED: 15016923] [DOI] [PubMed] [Google Scholar]
Neelon 1996
- Neelon VJ, Champagne MT, Calson JR, Funk SG. The NEECHAM confusion scale: construction, validation, and clinical testing. Nursing Research 1996;45(6):324‐30. [PUBMED: 8941300] [DOI] [PubMed] [Google Scholar]
Neufeld 2016
- Neufeld KJ, Yue J, Robinson TN, Inouye SK, Needham DM. Antipsychotic medication for prevention and treatment of delirium in hospitalized adults: a systematic review and meta‐analysis. Journal of the American Geriatrics Society 2016;64(4):705‐14. [PUBMED: 27004732 ] [DOI] [PMC free article] [PubMed] [Google Scholar]
OECD 2012
- Organization for Economic Co‐operation and Development. OECD Health Data. http://www.oecd.org/health/ (accessed 16 September 2014).
Ohta 2013
- Ohta T, Murao K, Miyake K, Takemoto K. Melatonin receptor agonists for treating delirium in elderly patients with acute stroke. Journal of Stroke and Cerebrovascular Diseases 2013;22(7):1107–10. [PUBMED: 23017429] [DOI] [PubMed] [Google Scholar]
Partridge 2013
- Partridge JS, Martin FC, Harari D, Dhesi JK. The delirium experience: what is the effect on patients, relatives and staff and what can be done to modify this?. International Journal of Geriatric Psychiatry 2013;28(8):804‐12. [PUBMED: 23112139 ] [DOI] [PubMed] [Google Scholar]
Patel 2014
- Patel SB, Poston JT, Pohlman A, Hall JB, Kress JP. Rapidly reversible, sedation‐related delirium versus persistent delirium in the intensive care unit. American Journal of Respiratory and Critical Care Medicine 2014;189(6):658‐65. [PUBMED: 24423152 ] [DOI] [PubMed] [Google Scholar]
Pisani 2009
- Pisani MA, Kong SY, Kasl SV, Murphy TE, Araujo KL, Ness PH, et al. Days of delirium are associated with 1‐year mortality in an older intensive care unit population. American Journal of Respiratory and Critical Care Medicine 2009;180(11):1092‐7. [PUBMED: 19745202 ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Reade 2014
- Reade MC, Finfer S. Sedation and delirium in the intensive care unit. New England Journal of Medicine 2014;370(5):444‐54. [PUBMED: 24476433] [DOI] [PubMed] [Google Scholar]
Review Manager 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Rose 2016
- Rose L, Burry L, Mallick R, Luk E, Cook D, Fergusson D, et al. Prevalence, risk factors, and outcomes associated with physical restraint use in mechanically ventilated adults. Journal of Critical Care 2016;31(1):31‐5. [PUBMED: 26489482] [DOI] [PubMed] [Google Scholar]
Rose 2017
- Rose L, Agar M, Burry L, Campbell N, Clarke M, Lee J, et al. Development of core outcome sets for effectiveness trials of interventions to prevent and/or treat delirium (Del‐ COrS): study protocol. BMJ Open 2017;7(9):e016371. [PUBMED: 28928181 ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Rosenbloom‐Brunton 2010
- Rosenbloom‐Brunton DA, Henneman EA, Inouye SK. Feasibility of family participation in a delirium prevention program for hospitalized older adults. Journal of Gerontological Nursing 2010;36(9):22‐33. [PUBMED: 20438016 ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Rubino 2010
- Rubino AS, Onorati F, Caroleo S, Galato E, Nucera S, Amantea B, et al. Impact of clonidine administration on delirium and related respiratory weaning after surgical correction of acute type‐A aortic dissection: results of a pilot study. Interactive Cardiovascular and Thoracic Surgery 2010;10(1):58‐62. [PUBMED: 19854793] [DOI] [PubMed] [Google Scholar]
Rudolph 2008
- Rudolph JL, Ramlawi B, Kuchel GA, McElhaney JE, Xie D, Sellke FW, et al. Chemokines are associated with delirium after cardiac surgery. Journals of Gerontology. Series A, Biological Sciences and Medical Sciences 2008;63(2):184‐9. [PUBMED: 18314455 ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Salanti 2011
- Salanti G, Ades A, Ioannidis J. Graphical methods and numerical summaries for presenting results from multiple‐treatment meta‐analysis: an overview and tutorial. Journal of Clinical Epidemiology 2011;64(2):163‐71. [PUBMED: 20688472 ] [DOI] [PubMed] [Google Scholar]
Salanti 2014
- Salanti G, Giovane C, Chaimani A, Caldwell DM, Higgins JPT. Evaluating the quality of evidence from a network meta‐analysis. PLoS One 2014;9(7):e99682. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sampson 2009
- Sampson M, McGowan J, Cogo E, Grimshaw J, Moher Lefebvre C. An evidence‐based practice guideline for the peer review of electronic search strategies. Journal of Clinical Epidemiology 2009;62(9):944‐52. [PUBMED: 19230612] [DOI] [PubMed] [Google Scholar]
Schunemann 2011
- Schunemann H, Oxman A, Higgins J, Vist G, Glasziou P, Guyatt G, editor(s). Chapter 11. Presenting results and 'Summary of findings' tables. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Schweickert 2009
- Schweickert WD, Pohlman MC, Pohlman AS, Nigos C, Pawlik AJ, Esbrook CL, et al. Early physical and occupational therapy in mechanically ventilated, critically ill patients: a randomised controlled trial. Lancet 2009;373(9678):1874‐82. [PUBMED: 19446324] [DOI] [PMC free article] [PubMed] [Google Scholar]
Spiegelhalter 2002
- Spiegelhalter DJ, Best NG, Carlin BP, Linde A. Bayesian measures of model complexity and fit. Journal of the Royal Statistical Society: Series B (Statistical Methodology) 2002;64(4):583‐639. [DOI: 10.1111/1467-9868.00353] [DOI] [Google Scholar]
Spiegelhalter 2014 [Computer program]
- Spiegelhalter D, Thomas A, Best N, Lunn D. OpenBUGS user manual (version 3.2.3). Cambridge, UK: Univeristy of Cambridge, March 2014.
Tropea 2009
- Tropea J, Slee J, Holmes AC, Gorelik A, Brand CA. Use of antipsychotic medications for the management of delirium: an audit of current practice in the acute care setting. International Psychogeriatrics 2009;21(1):172‐9. [PUBMED: 18983720] [DOI] [PubMed] [Google Scholar]
Trzepacz 2001
- Trzepacz PT, Mittal D, Torres R, Kanary K, Norton J, Jimerson N. Validation of the Delirium Rating Scale‐revised‐98: comparison with the delirium rating scale and the cognitive test for delirium. Journal of Neuropsychiatry and Clinical Neurosciences 2001;13(2):229‐42. [PUBMED: 11449030] [DOI] [PubMed] [Google Scholar]
Van den Boogaard 2012
- Boogaard M, Schoonhoven L, Evers AW, Hoeven JG, Achterberg T, Pickkers P. Delirium in critically ill patients: impact on long‐term health related quality of life and cognitive functioning. Critical Care Medicine 2012;40(1):112‐8. [PUBMED: 21926597 ] [DOI] [PubMed] [Google Scholar]
Wan 2014
- Wan X, Wang W, Liu J, Tong T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Medical Research Methodology 2014;14(1):135. [PUBMED: 25524443] [DOI] [PMC free article] [PubMed] [Google Scholar]
Wang 2005
- Wang PS, Schneeweiss S, Avorn J, Fischer MA, Mogun H, Solomon DH, et al. Risk of death in elderly users of conventional vs. atypical antipsychotic medications. New England Journal of Medicine 2005;353(22):2335‐41. [PUBMED: 16319382 ] [DOI] [PubMed] [Google Scholar]
White 2002
- White S. The neuropathogenesis of delirium. Reviews in Clinical Gerontology 2002;12(1):62‐7. [Google Scholar]
WHO Regional Office 2012
- World Health Organization. WHO Regional Office for Europe, European hospital morbidity database. http://data.euro.who.int/hmdb/index.php (accessed 16 October 2014).
Yepes‐Nunez 2019
- Yepes‐Nuñez JJ, Li SA, Guyatt G, Jack SM, Brozek JL, Beyene J, et al. Development of the summary of findings table for network meta‐analysis. Journal of Clinical Epidemiology 2019;115:1‐13. [DOI] [PubMed] [Google Scholar]
Zaal 2015
- Zaal IJ, Devlin JW, Peelen LM, Slooter AJ. A systematic review of risk factors for delirium in the ICU. Critical Care Medicine 2015;43:40‐7. [PUBMED: 25251759 ] [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Burry 2015
- Burry L, Mehta S, Williamson DR, Hutton B, Ely EW, Adhikari NKJ, et al. Pharmacological interventions for the treatment of delirium in critically ill patients. Cochrane Database of Systematic Reviews 2015, Issue 6. [DOI: 10.1002/14651858.CD011749] [DOI] [PMC free article] [PubMed] [Google Scholar]


