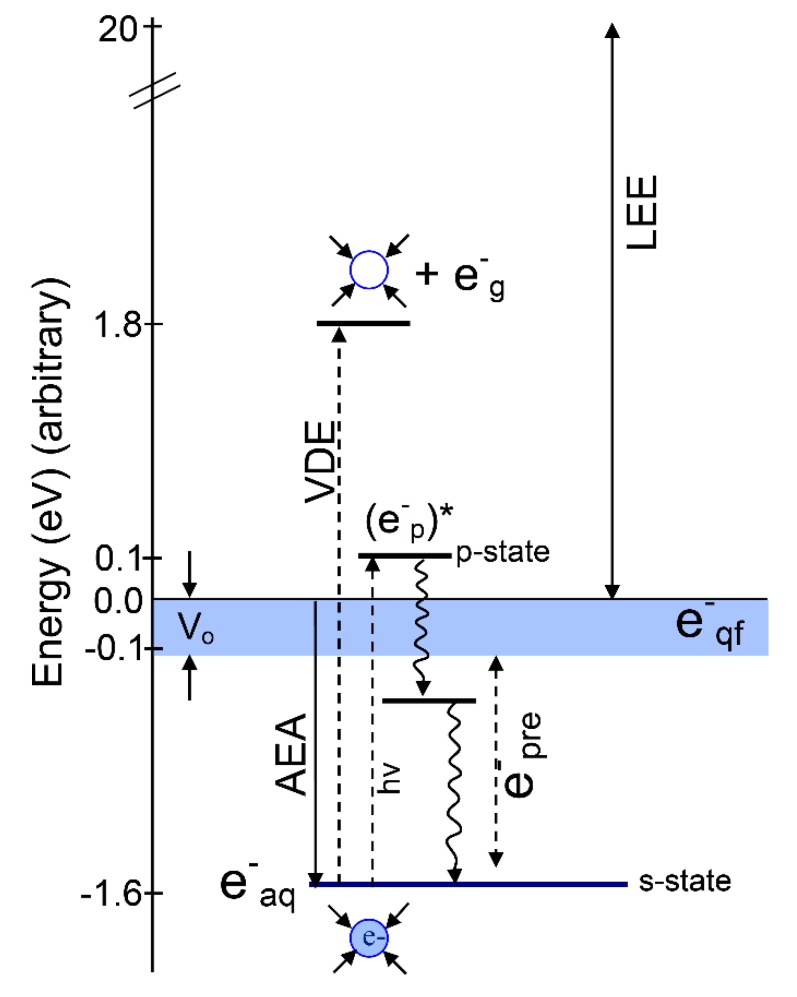
Scheme 1.
Energy diagram showing the addition of an electron to bulk water. The various energy values were taken from experiment. Adiabatic electron affinity (AEA, 1.6 eV) equals the negative of the free energy of solvation [32,33]. The vertical detachment energy (VDE) = 3.4 eV. Vo is taken from the experiment by Coe et al. [25]. The difference between VDE and AEA gives the relaxation energy (1.8 eV). The vertical excitation energy s→p* (1.73 eV) is taken from [34,35]. The fast solvent relaxation around (e−p)* within 200 fs before internal conversion is shown as e−pre which lies below the conduction band (blue band). e−pre lies in the energy range 0 eV to −1.6 eV which is between e−qf and e−aq (blue circle). The excited state energy levels of e−aq are based on those in reference [10]. Note that the zero of energy has the electron at zero eV and equilibrated (relaxed) water; e−g refers to a zero-eV gas phase electron. LEE are low energy electrons.