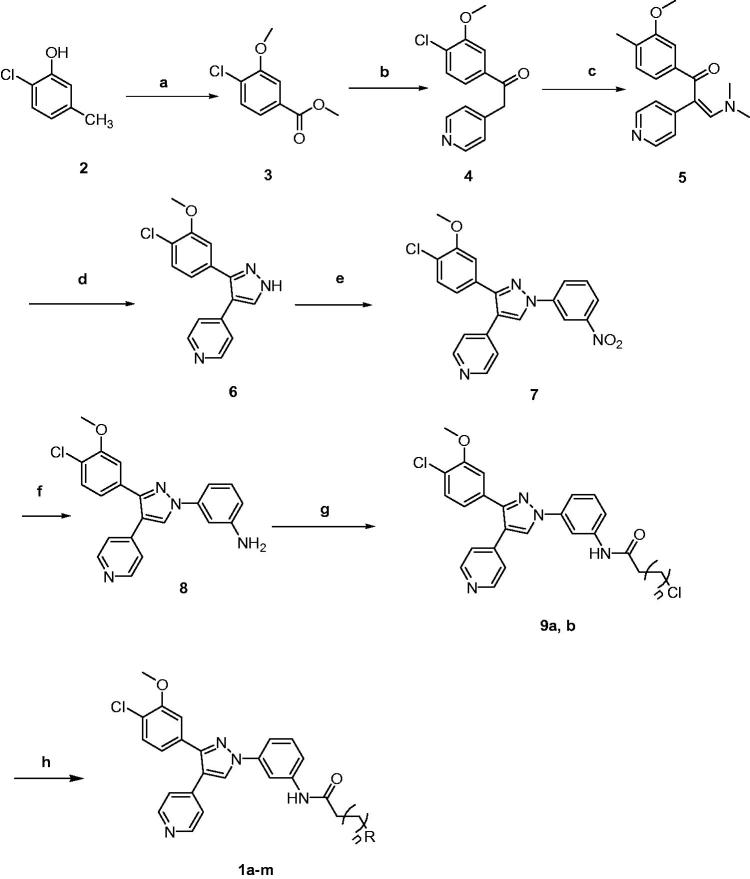
Scheme 1.
Reagents and conditions: (a) i) (CH3)2SO4, K2CO3, acetone, reflux, 1 h; ii) KMnO4, C5H5N, H2O, 50 °C, 24 h, then rt, 13 h; iii) acetyl chloride, CH3OH, rt, 15 h; (b) 4-picoline, LiHMDS, THF, rt, overnight; (c) DMF-DMA, rt, 18 h; (d) hydrazine monohydrate, C2H5OH, rt, overnight; (e) 1-iodo-4-nitrobenzene, K2CO3, CuI, L-proline, DMSO, 90 °C, 8 h; (f) H2, Pd/C, THF, rt, 2 h; (g) chloroacetyl chloride, or chloropropionyl chloride, TEA, CH2Cl2, −10 °C, 15 min; (h) appropriate amine derivative, TEA, CH2Cl2, rt, 1 h.