Figure 1.
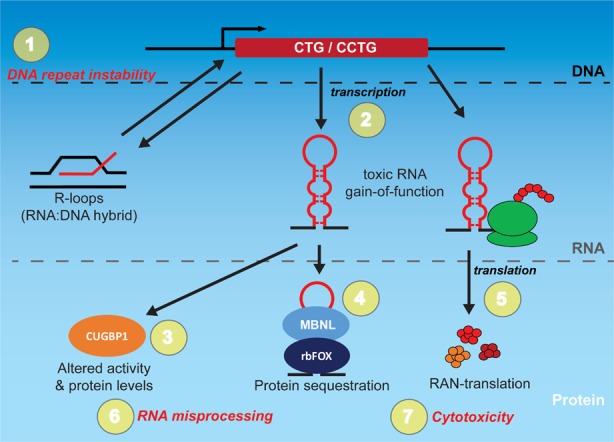
Multiple levels of disease mechanism and therapeutic targets in myotonic dystrophy (DM). A simplified schematic with both RNA gain-of-function (GOF) and repeat-associated non-ATG (RAN) translation protein products is illustrated. The specific proteins and processes vary between sense and antisense transcripts and between myotonic dystrophy type 1 (DM1) and 2 (DM2), with a generic process shown for review processes. Repeat expansion in the DNA is the source of numerous downstream pathogenic processes. Bi-directional transcription of CTG and CCTG repeat expansions produce toxic sense (CUG/CCUG) and antisense (CAG/CAGG) expansion RNA that can fold into hairpin structures with pathogenic downstream consequences. Co-transcriptional R-loops (a type of RNA:DNA hybrid) can trigger DNA repeat instability [1,2,3] which is positively correlated with increased disease severity. Toxic expansion RNAs can sequester important proteins, such as the MBNL and rbFOX family members [4], and trigger hyperphosphorylation of CUGBP1 leading to its increased steady state levels and altered activity. Toxic sense and antisense RNA can also trigger repeat-associated non-ATG (RAN) translation in multiple reading frames, producing toxic aggregating peptides that lead to cytotoxicity. Each of these different stages (expansion DNA, RNA, and protein) and their associated defects (1–7) represent a potential target of small molecules with unique advantages and disadvantages. Targeting the DNA repeat expansion (1) using small molecules for deletion or contraction should theoretically eliminate all downstream pathogenic process. While this type of treatment could also involve a single or few doses to achieve a permanent effect, it is technically challenging and there is little current progress on this front. Compounds that target transcription (2) may also alleviate many of the downstream consequences at lower doses compared with approaches that target the toxic RNA molecules itself, but will require continuous administration. Most progress in small molecule therapeutics for DM to date has been from targeting specific downstream consequences (3–7). While there are multiple targets, this approach is the furthest developed with several compounds showing considerable efficacy and progress in clinical trials. For example, there is a current phase II clinical trial with Tideglusib that modulates CUGBP1 activity.
