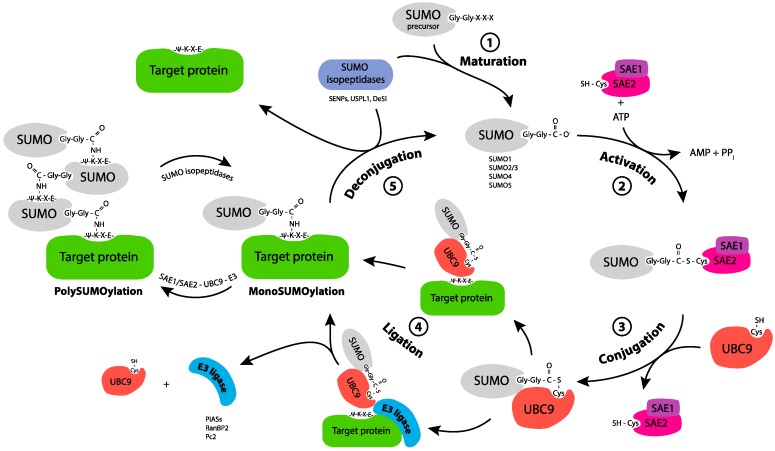
Figure 1.
SUMO conjugation/deconjugation cycle. SUMO-1, -2, -3 precursors are maturated through the action of SUMO isopeptidases, which cleave the C-terminus of SUMO to reveal a Gly-Gly motif (1. Maturation). Mature SUMO is activated by forming an ATP-dependent thioester bond between its C terminal Glycine and the catalytic Cys of the E1 enzyme, the heterodimer SAE1/SAE2 (2. Activation). Activated SUMO is transferred, through trans-thiolation, to the catalytic cysteine of the E2 enzyme, Ubc9 (3. Conjugation). Ubc9 triggers the enzymatic transfer to the Lys (K) of the target protein, either alone or with the help of a SUMO E3 ligase through the formation of an isopeptide bond (4. Ligation). The target protein can be monoSUMOylated, multi and/or polySUMOylated. SUMO isopeptidases cleave SUMO from its substrate and thereby release free SUMO (5. Deconjugation).