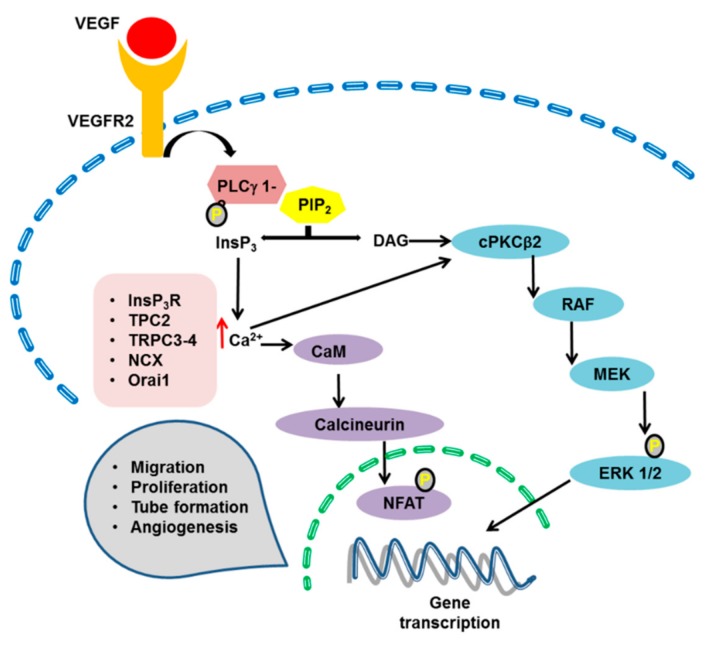
Figure 2.
Ca2+-dependent activation of the extracellular signal-regulated kinase (ERK) pathway and of the Nuclear Factor of Activated T-cells (NFAT). VEGF binding to VEGFR2 triggers an increase in intracellular Ca2+ concentration that may stimulate the ERK1/2 phosphorylation cascade or NFAT nuclear translocation. VEGF-induced endothelial Ca2+ signals may be mediated by multiple Ca2+-entry/release pathways, depending on species and vascular bed. The Ca2+ response to VEGF may recruit the Ca2+-dependent PKCβ2 (cPKCβ2), which engages the downstream RAF1–MEK–ERK1/2 cascade to induce gene expression. An increase in [Ca2+]i is required to promote cPKCβ2 translocation to the plasma membrane, where it is activated by DAG. Moreover, VEGF-induced endothelial Ca2+ signals may be sensed by calmodulin (CaM), which in turn activates calcineurin to dephosphorylate NFAT, thereby inducing its nuclear translocation. See Figure 1, Section 4.1 (ERK) and Section 4.3 (calcineurin and NFAT) for further details.