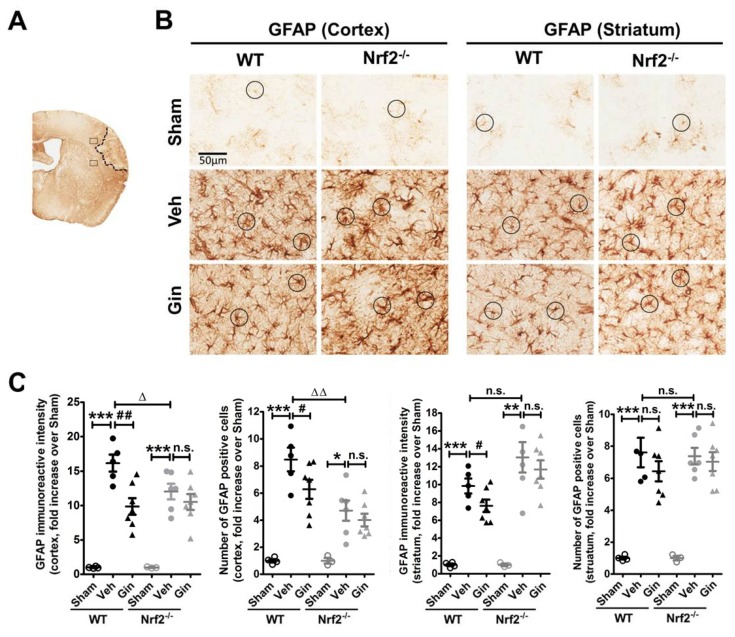
Figure 2.
The Nrf2-associated reactive astrogliosis contributes to ginseng neuroprotection. (A) Schematic representation of the GFAP staining and the infarct area; the squares indicate the peri-infarct areas of the cortex and striatum for micrographic measurement. (B) The representative images of GFAP-positive astrocytes in the peri-infarct areas of the cortex and striatum. The round boxes indicate the typical morphology of astrocytes. (C) Quantitative analysis revealed the overall GFAP immunoreactive intensity and the total number of GFAP-positive cells in the cortex and striatum. In the peri-infarct cortex areas of both genotypes of mice, ischemia evoked extremely higher reactive astrogliosis, indicated by either the overall immunoreactive intensity or the total number of astrocytes, which was more severe in Nrf2−/− mice. In contrast, ginseng significantly attenuated the ischemia-induced increase of reactive astrogliosis in the WT but not the Nrf2−/− mice. Interestingly, no significant difference was detected in the striatum among post-pdMCAO groups, except that ginseng reduced the overall GFAP reactive intensity. * p < 0.05, ** p < 0.01, *** p < 0.001, ∆ p < 0.05, ∆∆ p < 0.01. n = 3–4 per sham group, n = 5–7 per ischemic group. pdMCAO: permanent distal middle cerebral artery occlusion; GFAP: glial fibrillary acidic protein; Gin: Ginseng; Veh: Vehicle; n.s.: no significance.