Abstract
We report a case of primary pulmonary cryptococcosis in a 59-year-old female patient with a history of systemic lupus erythematosus, interstitial lung disease and glaucoma. She presented with a cough, severe fatigue, unintentional weight loss, shortness of breath (increase in home oxygen use from baseline) and pleuritic chest pain of 2 months duration. During these 2 months, her symptoms had worsened despite multiple hospital visits, empirical antibiotics and empirical increase of her steroid dosage. Cytopathology of the bronchoalveolar lavage fluid showed yeast cells with narrow-based budding and grew Cryptococcus neoformans on fungal culture. She was treated with oral fluconazole 400 mg/day for 6 months with an improvement in cough, decrease in shortness of breath (return to baseline oxygen use) and resolution of pleuritic chest pain.
Keywords: pneumonia (infectious disease), rheumatology, systemic lupus erythematosus, cryptococcus, pathology
Background
Humans become infected by cryptococcus by inhalation of fungal spores. Although most immunocompetent individuals infected by cryptococcus remain asymptomatic, it is a significant cause of disease in immunocompromised patients.1 Despite being a common opportunistic infection in immunocompromised patients, the diagnosis could be delayed due to the insidious onset of non-specific symptoms or confounding underlying medical conditions such as interstitial lung disease (ILD), chronic obstructive pulmonary disease or pulmonary fibrosis. This is an account of one such patient who was unavailingly treated with oral antibiotics and increased doses of immunosuppression before the identification of pulmonary cryptococcosis as the actual cause of her worsening respiratory symptoms.
Case presentation
A 59-year-old woman with a 30-year history of systemic lupus erythematosus (SLE) and glaucoma developed a dry cough in March 2018. Over the next month, she developed a non-pruritic rash on her face and chest along with increased fatigue. She also noticed that her voice had become feeble. She presented to a local hospital where a presumptive diagnosis of pneumonia was made, and she was treated with 5 days of empirical azithromycin. An ENT examination at that time showed left vocal fold atrophy of unknown aetiology and she was recommended speech therapy and vocal fold bulking injections. Despite antibiotic therapy, all of her symptoms persisted.
She presented to a different hospital a month later, where a trial of increased dose of prednisone (60 mg/day which was gradually tapered to 20 mg/day) was initiated for a possible lupus flare. A pulmonologist evaluated the patient in the outpatient setting due to ongoing symptoms and diagnosed her with ILD based on radiological appearance. After the diagnosis of ILD, plaquenil was added with no improvement in symptoms.
In the following 2 months, the patient visited many different hospitals for worsening shortness of breath, fatigue and cough. She continued to have decreased appetite despite the steroid therapy and lost 14 kg of weight in 3 months. Her symptoms did not improve with empirical antibiotics, plaquenil and increasing doses of prednisone. It is unclear if markers such as C3 and C4 were obtained for acute lupus flare at these hospitalisations before increasing the steroid dose.
Four months after the onset of her cough, the patient presented to our hospital with exertional shortness of breath, non-productive cough, fatigue and pleuritic chest pain that had not improved with plaquenil, steroids and multiple courses of empirical antibiotics (azithromycin, vancomycin, piperacillin with tazobactam and levofloxacin). She noted that 2 months ago when she was diagnosed with ILD, she used 2 L/min of oxygen through nasal cannula only as needed during exertion; however, she now needed 3 L/min even at rest. The patient did not have any pets and denied any significant exposure to birds. On admission, her oxygen saturation was 98% on 3 L/min of oxygen through a nasal cannula.
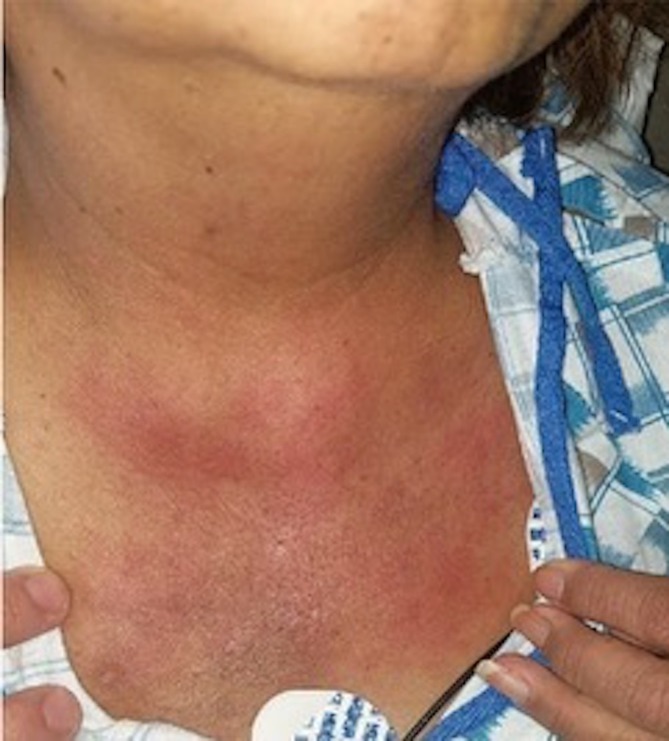
On physical examination, the patient was tachypneic despite being on 3 L/min of oxygen through the nasal cannula. She was noted to have a mildly Cushingoid appearance and alopecia. There was a long-standing hyperpigmented rash on her forehead, around her eyebrows and right periorbital region. There was also an erythematous blanching rash on her upper chest (figure 1) and hyperpigmentation of the elbows bilaterally.
Figure 1.
Erythematous blanching rash on patient’s upper chest.
Her pulse was 76 beats/min; blood pressure was 101/80 mm Hg; her oral temperature was 36.8°C ; body mass index was 29.27 kg/m2 and SpO2 with 3 L/min of supplemental oxygen was 98%. The pulmonary examination was notable for bilateral basilar crackles, but her physical exam was otherwise unremarkable.
Investigations
Blood counts showed a white blood cell count of 4.16×109/L, haemoglobin 10.5 g/dL with normocytic red blood cells and platelets 219×109 /L. Liver function tests showed aspartate aminotransferase: 64 U/L (5.0–34 U/L), alanine aminotransferase: 88 U/L (5–55 U/L), alkaline phosphatase: 76 U/L (40–150 U/L). Kidney function tests were within normal limits. C3 level was 122 mg/dL (83–193 mg/dL) and C4 level was 32.2 mg/dL (15–57 mg/dL).
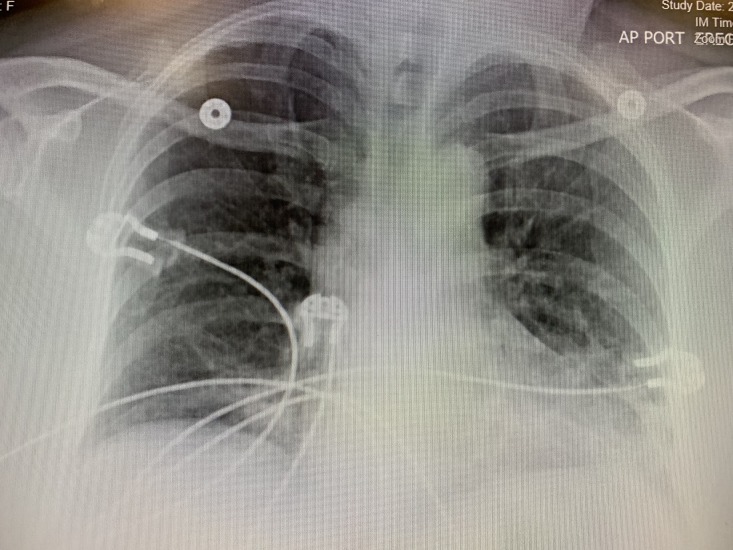
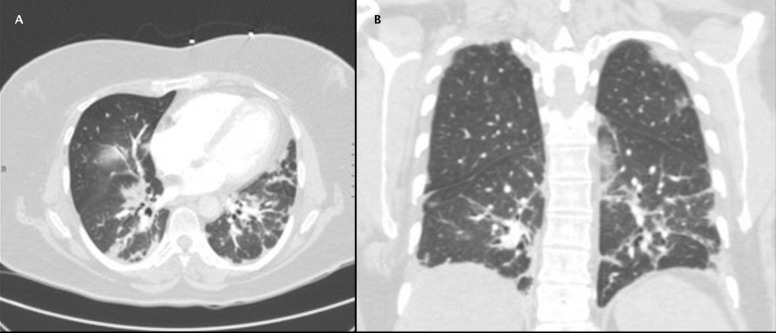
Chest X-ray showed bilateral reticular opacities (figure 2). Chest CT showed no evidence for pulmonary embolus but showed subpleural reticulation thought to be related to the patient’s history of ILD (figure 3). Hepatic steatosis was also noted on CT. Pulmonary function tests (PFTs) performed 1 month prior to the admission were significant for moderate restriction with severely decreased diffusion lung capacity for carbon monoxide (DLCO), forced vital capacity (FVC) of 44% of predicted value (pred.), forced expiratory volume in 1 s (FEV1) of 51% pred., FEV1/FVC=85% pred., total lung capacity (TLC) of 61% pred., residual volume (RV) of 80% pred. and DLCO of 50% pred.
Figure 2.
Anterioposterior view of the chest X-ray showing bilateral reticular opacities.
Figure 3.
Axial (A) and coronal (B) views of contrast-enhanced CT chest showing bilateral reticular opacities.
A bronchoscopy was performed, and bronchoalveolar lavage fluid was obtained for cytopathology and culture. Respiratory viral PCR panel, including adenovirus, rhinovirus, respiratory syncytial virus, influenza A and B, herpes simplex virus and human metapneumovirus, was negative. Sputum smear was negative for acid-fast bacilli, legionella and nocardia. HIV antigen and antibody assays and pneumocystis pneumonia (PCP) cytology turned out negative. β-D-glucan and galactomannan were investigated for invasive aspergillosis and both the tests were negative.
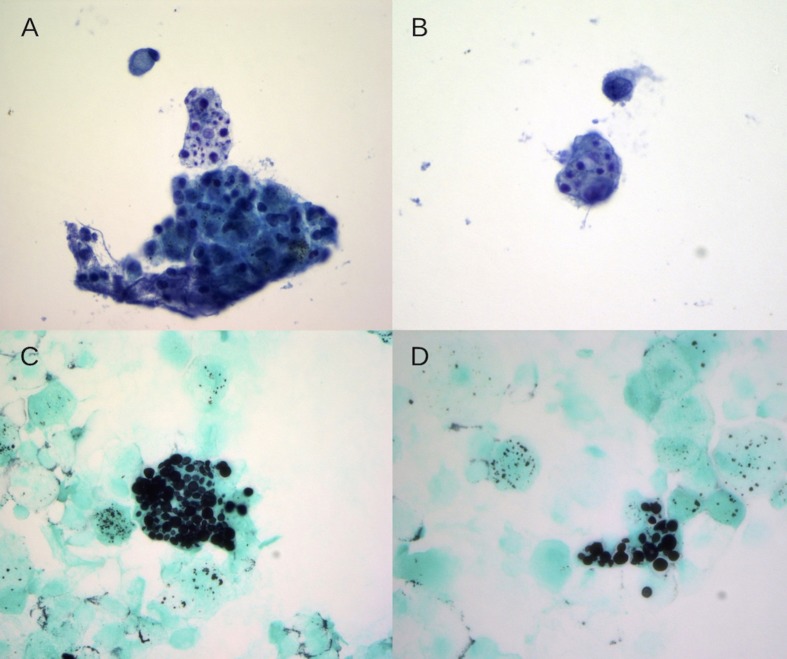
Cytology of the bronchoalveolar lavage fluid showed fungal yeasts with narrow budding within pulmonary macrophages (figure 4). Fungal cultures grew Cryptococcus neoformans, and the diagnosis of cryptococcal pneumonia was made. Fungal blood culture and serum cryptococcal antigen were negative. A lumbar puncture was advised but the patient refused the procedure.
Figure 4.
Bronchoalveolar lavage cytology specimen demonstrating variably sized round to oval intracellular fungal yeast forms, measuring between 4 and 10 µm with narrow-based budding, present within pulmonary macrophages ((A, B) Papanicolaou stain, ThinPrep, 600×; (C, D) Grocott methenamine silver stain, Cytospin, 600×)).
Differential diagnosis
Fungal pneumonia caused by histoplasma, coccidioides or PCP was in the differential diagnosis. Other considerations were Mycobacterium tuberculosis, drug-induced pneumonitis versus lupus flare.
Treatment
Itraconazole was started empirically while awaiting results of the fungal culture. Antifungal therapy was changed to oral fluconazole 400 mg/day for 6 months after cultures grew C. neoformans. The patient was also started on PCP prophylaxis.
Outcome and follow-up
The patient has completed 6 months of oral fluconazole and has tolerated the treatment well. Her respiratory symptoms have improved to her baseline, requiring oxygen intermittently during exertion rather than being oxygen-dependent at all times. The recovery has allowed the patient to be ambulatory which was her priority. She is currently being followed up by her pulmonologist. Most recently, on her follow-up visit in April 2019, her saturation was noted to be 98% on 2 L/min of oxygen on exertion. On follow-up, her PFT showed an FVC of 42% pred., FEV1 of 45% pred., FEV1/FVC=107% pred., TLC of 60% pred. RV of 88% pred. and DLCO of 28% pred.
Discussion
Cryptococci are encapsulated yeasts with a global distribution. It is a common opportunistic infection in the immunocompromised, especially in HIV-infected patients.2 C. neoformans and C.gatti are the important disease-causing species. Human infections often occur in immunocompromised patients; however, C. gatti has been attributed to outbreaks in immunocompetent individuals, particularly in tropical and subtropical environments.3 4
Cryptococcal infection is acquired via the lungs, but due to the neurotropic nature of the fungus, the brain and the meninges are commonly affected.4 Clinical presentation varies from asymptomatic to severe life-threatening disease based on the affected organ and the immune status of the patient.4 Most immunocompromised patients with isolated pulmonary disease are symptomatic with fever, cough and dyspnoea.5 These patients may also have alveolar, interstitial or nodular infiltrates usually associated with hilar lymphadenopathy.5 Skin lesions and occasional pleural effusions have also been reported along with the above findings. Immunocompetent patients, on the other hand, can be asymptomatic, have subtle symptoms like a subacute or chronic cough or may present with an isolated pulmonary nodule.6
In our patient, the presence of SLE and ILD confounded the clinical picture. Her dyspnoea was initially thought to be related to a lupus flare and then to ILD. Both lupus flare and ILD could have been potential contributors to the patient’s dyspnoea; nevertheless, a careful investigation for opportunistic infections such as M. tuberculosis, histoplasmosis, pneumocystis pneumonia, coccidioides and cryptococcosis should be considered in immunocompromised patients. A careful examination of the bronchoalveolar lavage cytology helped to initiate antifungal treatment much earlier in the course than would have been possible with fungal cultures alone. Radiographical findings such as pulmonary nodules, cavitation and granulomas are supportive but non-specific in such cases.7 Although pulmonary cryptococcosis can often have an indolent course, it carries a significant risk of disseminated infection or meningoencephalitis which can be fatal.5 A high index of suspicion is thus crucial to avert a catastrophic outcome.
Patient’s perspective.
My bigger concern after the cryptococcal infection was quality of life. I was willing to try the medication with least side effects that would not worsen my quality of life. It has been challenging dealing with hair loss and breathing problems due to lupus, but I did not want more procedures or medications that would cause more side effects without much improvement in my day-to-day life. The treatment for my cryptococcal infection did help me to decrease my oxygen use which helps me be more mobile.
Learning points.
In patients with an autoimmune disease, typical symptoms and signs of infection might be absent and could be confounded by underlying comorbidities. Hence, a strong index of suspicion for opportunistic infection such as pulmonary cryptococcosis is essential.
Since the differential diagnosis could be vast, an early attempt should be made to obtain a tissue diagnosis. A careful examination of the bronchoalveolar fluid during cytopathology is vital for early diagnosis as the growth of fungal culture could be delayed.
In the immunocompromised patients, it is essential to expediently make a definitive diagnosis since patients can deteriorate very quickly. A close consultation with rheumatologist is necessary to optimise immunosuppression to the lowest feasible level.
This case also highlights constant patient engagement in shared decision making. The patient declined lumbar puncture despite a discussion regarding the associated risks versus benefits. She decided not to choose intravenous amphotericin for treatment due to the multitude of side effects even if she had cryptococcal involvement of her nervous system.
Footnotes
Contributors: All the three authors of the case report have contributed to the following areas. The names of the authors are mentioned in the order of their contribution to the individual areas. Drafting the work or revising it critically for important intellectual content: RG, SL, EO’N. Substantial contributions to the conception or design of the work, or the acquisition, analysis or interpretation of data: RG, EO’N, SL. Final approval of the version published: all the authors have contributed equally. The authors agree to be accountable for all aspects of the work. The authors also ensure that the questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Patient consent for publication: Obtained.
References
- 1. He Q, Ding Y, Zhou W, et al. . Clinical features of pulmonary cryptococcosis among patients with different levels of peripheral blood CD4+ T lymphocyte counts. BMC Infect Dis 2017;17:768 10.1186/s12879-017-2865-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Skolnik K, Huston S, Mody CH. Cryptococcal lung infections. Clin Chest Med 2017;38:451–64. 10.1016/j.ccm.2017.04.007 [DOI] [PubMed] [Google Scholar]
- 3. Zheng S, Tan TT, Chien JMF. Cryptococcus gattii infection presenting as an aggressive lung mass. Mycopathologia 2018;183:597–602. 10.1007/s11046-017-0233-6 [DOI] [PubMed] [Google Scholar]
- 4. Li SS, Mody CH. Cryptococcus. Proc Am Thorac Soc 2010;7:186–96. 10.1513/pats.200907-063AL [DOI] [PubMed] [Google Scholar]
- 5. Miller KD, Mican JA, Davey RT. Asymptomatic solitary pulmonary nodules due to Cryptococcus neoformans in patients infected with human immunodeficiency virus. Clin Infect Dis 1996;23:810–2. 10.1093/clinids/23.4.810 [DOI] [PubMed] [Google Scholar]
- 6. Chang CC, Sorrell TC, Chen SC. Pulmonary cryptococcosis. Semin Respir Crit Care Med 2015;36:681–91. 10.1055/s-0035-1562895 [DOI] [PubMed] [Google Scholar]
- 7. Deng H, Zhang J, Li J, et al. . Clinical features and radiological characteristics of pulmonary cryptococcosis. J Int Med Res 2018;46:2687–95. 10.1177/0300060518769541 [DOI] [PMC free article] [PubMed] [Google Scholar]