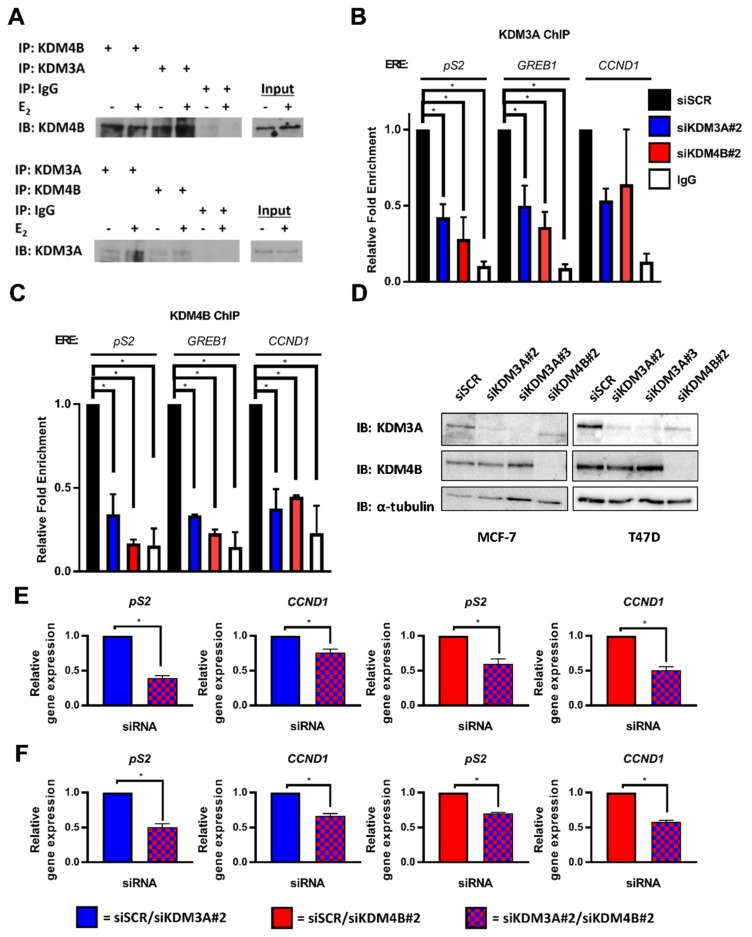
Figure 1.
KDM3A interacts with KDM4B and regulates chromatin occupancy. (A) MCF-7 cells grown in steroid-depleted (−E2) and 10 nM E2-supplemented (+E2) media were subject to immunoprecipitation (IP) using anti-KDM4B, anti-KDM3A, or isotype control (IgG) antibodies before western blot analysis (IB) using reciprocal antibodies. (B,C) MCF-7 cells were transiently transfected with either siSCR, siKDM3A#2, or siKDM4B#2 and grown in steroid-depleted conditions for 72 h prior to treatment with 10 nM E2 for 45 min followed by ChIP analysis using antibodies specific to KDM3A (B) or KDM4B (C) and isotype controls (IgG). ChIP using the isotype control antibody was performed on siSCR transfected cells in each experiment. Enrichment of KDM3A and KDM4B at pS2, GREB1, and CCND1 oestrogen response elements (EREs) was assessed by qPCR. Data are an average of 2 independent experiments ± SEM and are expressed relative to the level of enrichment measured in the siSCR transfected cells. P-values were determined by Dunnet’s multiple comparisons test (* denotes p < 0.05). (D) MCF-7 and T47D cells were transiently transfected with either siSCR, siKDM3A#2, siKDM3A#3, or siKDM4B#2 and grown in steroid-depleted conditions for 72 h prior to treatment with 10 nM E2 for 4 h and then western blot analysis using antibodies specific to KDM4B, KDM3A, and α-tubulin. α-tubulin was used to compare protein loading between samples. (E,F) pS2 and CCND1 gene expression in MCF-7 (E) or T47D (F) cells transfected with either an siRNA mixture of siSCR and siKDM3A#2 (red) or siSCR and siKDM4B#2 (blue) (single knockdowns) and compared with expression in cells transfected with an siRNA cocktail of siKDM3A#2 and siKDM4B#2 (red and blue) (dual knockdown). Cells were transfected and grown in steroid depleted media for 72 h prior to stimulation with E2 for 4 h and RNA extraction. qPCR data are an average of 3 repeats ± SEM and are expressed relative to gene expression in single gene knockdown cells. P values were determined by Students T test (* = p < 0.05).