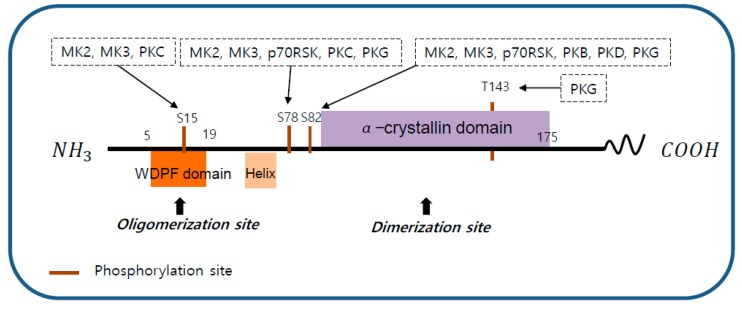
Figure 2.
The structure of heat shock protein 27 (HSP27). The structure of human HSP27 consists of the N-terminal domain, the α-crystallin domain, and the C-terminal domain. The N-terminal domain contains a WDPF motif which is essential for large oligomerization. The C-terminal domain includes an α-crystallin motif that is highly conserved between species and is involved in the formation of small oligomerization. HSP27 phosphorylation sites S15, S78, S82, and T143 are indicated. S15 can be phosphorylated by p38 mitogen-activated protein kinase (MAPK)-activated protein kinase 2 (MK2) and 3 (MK3), and protein kinase C (PKC). S78 can be phosphorylated by MK2, MK3, ribosomal S6 kinase (p70RSK), PKC, and protein kinase G (PKG). S82 can be phosphorylated by MK2, MK3, p70RSK, protein kinase B (PKB), protein kinase D (PKD), and PKG. T143 can be phosphorylated by PKG.