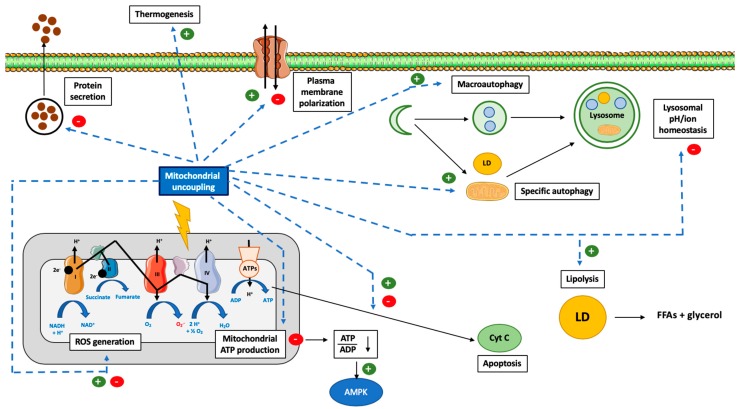
Figure 1.
Overview of the cellular consequences of mitochondrial uncoupling. Abbreviations: ADP (Adenosine DiPhosphate), AMPK (Adenosine Monophosphate-Activated Protein Kinase), ATP (Adenosine Triphosphate), ATPs (ATPsynthase), CytC (Cytochrome C), FFA (Free Fatty Acid), LD (Lipid Droplet), ROS (Reactive Oxygen Species), NAD (Nicotinamide Adenine Dinucleotide). Induction of mitochondrial uncoupling by use of synthetic or natural uncoupling agents or by activating dedicated proteins, such as UCPs or ANTs, can trigger several cellular mechanisms. The first effect, and the most well-known, is the inhibition of mitochondrial ATP production by ATP synthase due to the dissipation of the mitochondrial proton gradient. This energy will be dissipated as heat (thermogenesis). The decrease in cytosolic ATP will be sensed by AMPK and lead to its activation. In addition, the decrease in mitochondrial potential membrane will also modify ROS generation. In order to cope with this energy loss, autophagy will also be triggered (both bulk and specific forms of autophagy). Lipid droplets (LD) will be degraded by a form of autophagy and help to fuel the cell with lipids. Loss of the mitochondrial potential membrane will also allow the identification of these dysfunctional mitochondria by autophagy (mitophagy). Mitochondrial uncoupling can also help to protect cells against cell death and apoptosis but can also promote it, according to the cell type, mitochondrial uncoupler and mitochondrial uncoupling intensity considered. Finally, the use of non-specific mitochondrial uncouplers, such as FCCP or DNP, could alter the homeostasis of several ions, such as Ca2+, Na+ and K+ (at the cytosolic, mitochondria, or lysosomal levels), which will lead to a decrease in protein secretion and plasma membrane (de)polarization, respectively.