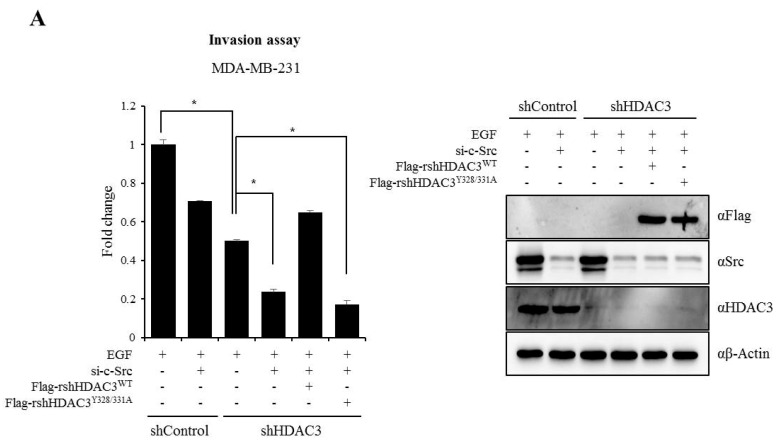
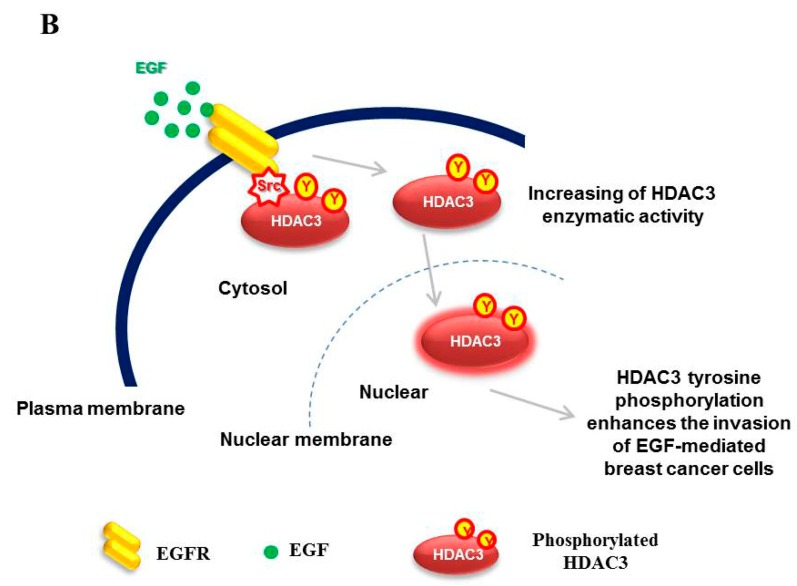
Figure 6.
c-Src-mediated HDAC3 phosphorylation enhances the invasion of breast cancer cells. (A) siRNA targeting c-Src was transiently transfected into either the sh-Control or sh-HDAC3-expressed stable MDA-MB-231 cells, and after 24 h, rsh-HDAC3WT or rsh-HDAC3Y328/331 was overexpressed for another 36 h. Cell invasion was measured using the Matrigel system, and results are presented as the fold of the control (left panel). Results are expressed as means ± SD calculated from three independent experiments. *P ≤ 0.05. Small interfering RAN against c-Src (si-c-Src), Flag-rsh-HDAC3wt, and Flag-rsh-HDAC3Y328/331 were validated with indicated antibodies using Western blot analysis (right panel). (B) Model of our findings. EGF stimulation triggers the formation of the EGFR–c-Src–HDAC3 heterocomplexes near the plasma membrane, induces the EGFR–c-Src-mediated phosphorylation of Y328 and Y331 on HDAC3, resulting in the induction of HDAC3 enzymatic activation. Phosphor-HDAC3Y328/331 was translocated into the nucleus. The association of EGFR–c-Src-HDAC3, consequentially, enhanced the invasiveness of breast cancer cells.