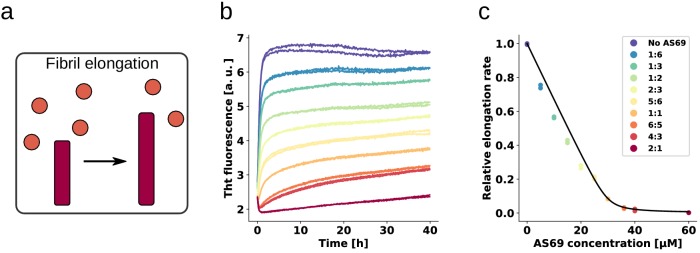
Figure 4. AS69 inhibits -synuclein fibril elongation.
(a) Schematic representations of fibril elongation. (b) Change in ThT fluorescence when a 30 μM solution of monomeric -synuclein was incubated in the presence of 5 μM pre-formed fibrils under quiescent conditions with increasing concentrations of AS69. (c) Relative rates of fibril elongation with increasing concentrations of AS69. The solid line corresponds to a prediction based on the affinity of AS69 for monomeric -synuclein (240 nM, Figure 1b [Mirecka et al., 2014], see Appendix 1 for details).