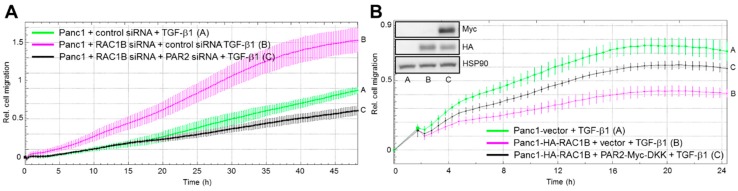
Figure 3.
Effect of PAR2 depletion or ectopic overexpression on TGF-β-regulated chemokinesis in Panc1 cells with RAC1B knockdown or stable overexpression, respectively. (A) Panc1 cells were transfected twice with 25 nM each of Co siRNAs for RAC1B and PAR2, or 25 nM each of RAC1B siRNA and PAR2-specific Co siRNA, or 25 nM RAC1B siRNA + 25 nM PAR2 siRNA. Forty-eight hours after the second round of transfection, cells were assayed for migratory activity on an xCELLigence platform in the presence of TGF-β1. The graph shows a representative experiment. Data are the mean ± SD from 3–4 wells per condition. Differences between Panc1 + RAC1B siRNA + PAR2 siRNA + TGF-β1 (black curve, tracing C) and Panc1 + RAC1B siRNA + TGF-β1 (magenta curve, tracing B) are significant at 01:00 and all later time points. Successful inhibition of RAC1B and PAR2 was verified by immunoblotting and qPCR analysis, respectively. (B) Panc1 cells with ectopic expression of HA-RAC1B (clone 4) were transiently transfected with Myc-DKK-tagged PAR2, or empty vector, and 48 h later subjected to real-time cell migration assay in the presence of TGF-β1. Panc1 cells with stable expression of empty pCGN vector (Panc1-vector) rather than HA-RAC1B were used as control for the migration-inhibitory effect of HA-RAC1B. Shown is a representative experiment (mean ± SD from 3–4 wells per condition). Differences between Panc1-HA-RAC1B + PAR2-Myc-DKK + TGF-β1 (black curve, tracing C) and Panc1-HA-RAC1B + empty vector + TGF-β1 (magenta curve, tracing B) are significant at 04:00 and all later time points. Ectopic expression of HA-RAC1B and PAR2-Myc-DKK were verified in immunoblots using anti-HA and anti-Myc antibodies, respectively (inset).