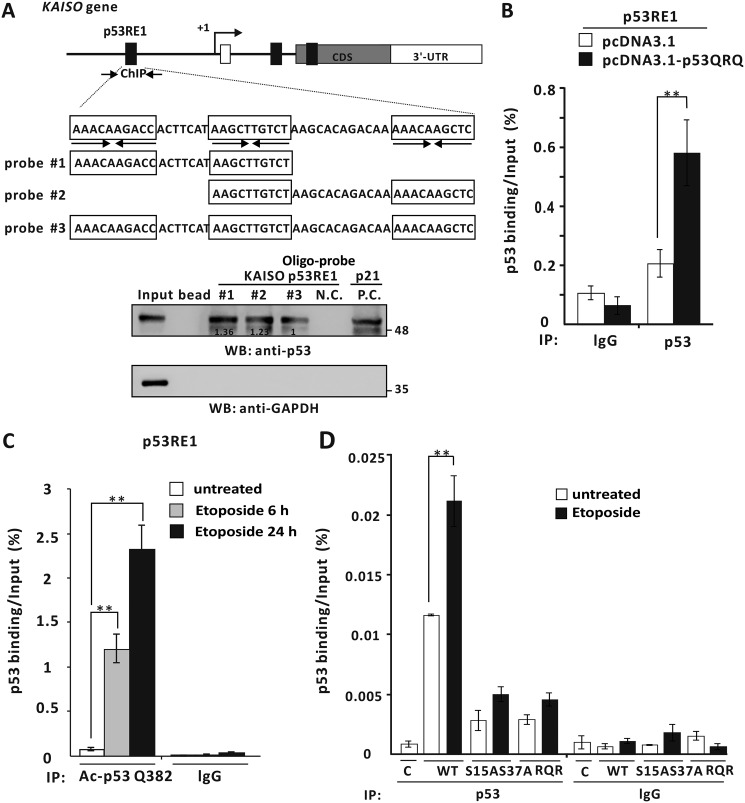
Figure 9.
The mimic of a KAISO-mediated acetylated p53, p53QRQ, binds to the p53RE1 of the 5′-upstream regulatory region of KAISO. A, structure and oligonucleotide pulldown/Western blotting assay (WB) of p53RE1 of the KAISO gene. p53RE1 contains three p53-binding half-sites. The locations of oligonucleotide primer–binding sites of the ChIP assay of p53QRQ binding are indicated by arrows. Probes #1 to #3 used in oligonucleotide pulldown assays are indicated below. Oligonucleotide pulldown and Western blotting assays of p53QRQ binding to p53RE1 probes are shown. H1299 p53-null cells extracts with ectopic p53QRQ were incubated with biotinylated double-stranded oligonucleotides. The mixtures were further incubated with streptavidin-agarose beads and precipitated by centrifugation. The precipitate was analyzed by a Western blotting assay using antibodies against p53 (DO-1) or GAPDH. p53QRQ bound similarly to all three probes. 3′-UTR was used as negative control. p53RE1 of the p21/CDKN1A gene was positive control. B, ChIP assay of p53QRQ binding to the p53RE1 of the endogenous KAISO gene in the cells transfected with pcDNA3.1-p53QRQ. IP, immunoprecipitation. C, ChIP assays of endogenously acetylated p53-K382 binding to p53RE1 of endogenous KAISO. HCT116 p53+/+ cells treated with etoposide (60 μm) were harvested at 6 or 24 h, immunoprecipitated with a specific anti-acetylated p53 Lys-382 antibody, and amplified by PCR of the region flanking p53RE1. Shown is the average of three independent assays. Error bars, S.D. **, p < 0.05. D, PTMs of p53, including phosphorylation of Ser-15 and Ser-37 and acetylation of Lys-382, PTMs critical for binding the KAISO p53RE1. Dominant-negative forms of phosphorylated p53 at Ser-15 and/or Ser-37 (p53-S15A·S37A) or the KAISO-mediated acetylated form (p53RQR) were expressed in H1299 cells treated with etoposide (60 μm) and analyzed for p53 binding. The mutations strongly attenuated p53 binding.