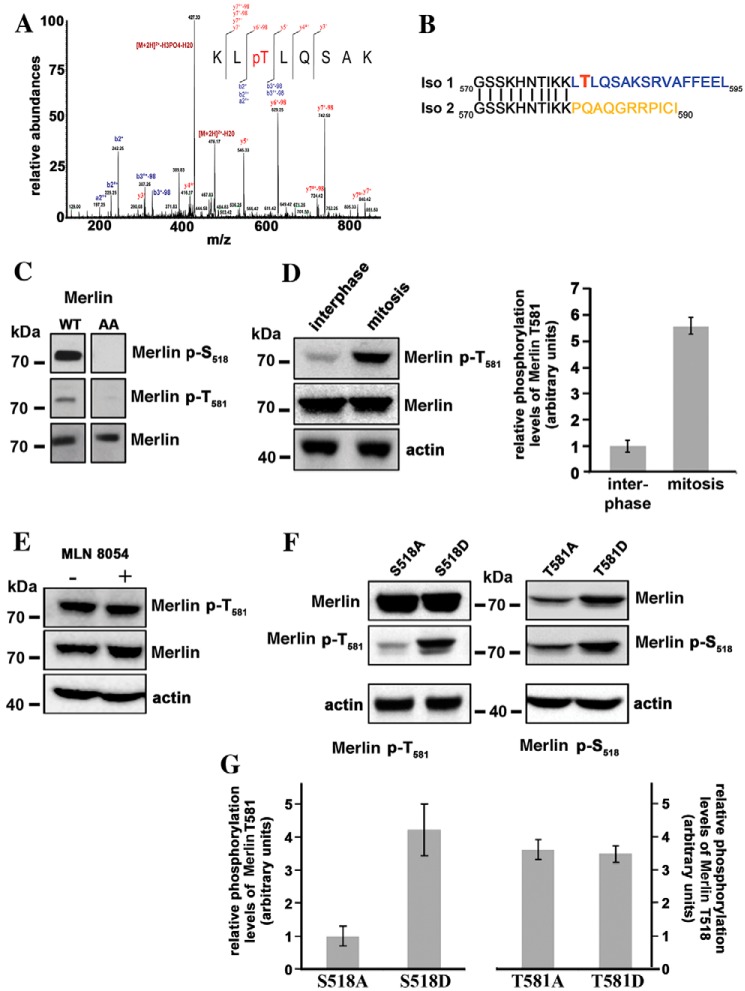
Figure 2.
Threonine 581 of Merlin isoform 1 is phosphorylated during mitosis. A, mass spectrometry analysis of HEK293 cells overexpressing WT Merlin isoform 1 identifies a peak corresponding to a peptide phosphorylated on threonine 581. The peptide sequence and the observed ions of the phosphopeptide are shown with the spectrum. MS/MS spectrum is labeled to show singly charged b and y ions, as well as ion corresponding to neutral losses of water (o), NH3 (*), and H3PO4 group (98 Da). LC-ESI MS/MS spectrum for peptide with the position of the phosphate group KLpT (581) LQSAK (484.762+ m/z). B, the comparison of Merlin isoforms 1 and 2 sequences C-terminal to amino acid 570 show that Thr-581 is unique to isoform 1. C, an antibody raised against a peptide including phospho–Thr-581 recognizes Merlin overexpressed in HEK293 but does not recognize Merlin S518/T581A mutant (AA), proving good specificity. Specificity of the antibody to Ser-518 was checked in parallel. D, Thr-581 is highly phosphorylated during mitosis in HeLa cells overexpressing Merlin. The quantification of three independent experiments is shown on the right panel. E, the treatment of mitotic HeLa cells with MLN8054 has no impact on Thr-581 phosphorylation. F, the influence of alanine and aspartic substitutions of Ser-518 or Thr-581 on the phosphorylation level of the other site was evaluated by Western blotting on extract from mitotic HeLa cells. The S518A substitution clearly reduces the phosphorylation of Thr-581 compared with S518D. There is no impact of T581A and D substitutions on Ser-518 phosphorylation levels. G, quantification of three independent experiments corresponding to the conditions used in panel F. The ratio of phosphorylated Merlin to total Merlin is presented. Actin was used as loading control. All blots shown above are representative of three independent biological experiments (n = 3). Error bars represent mean ± S.D.