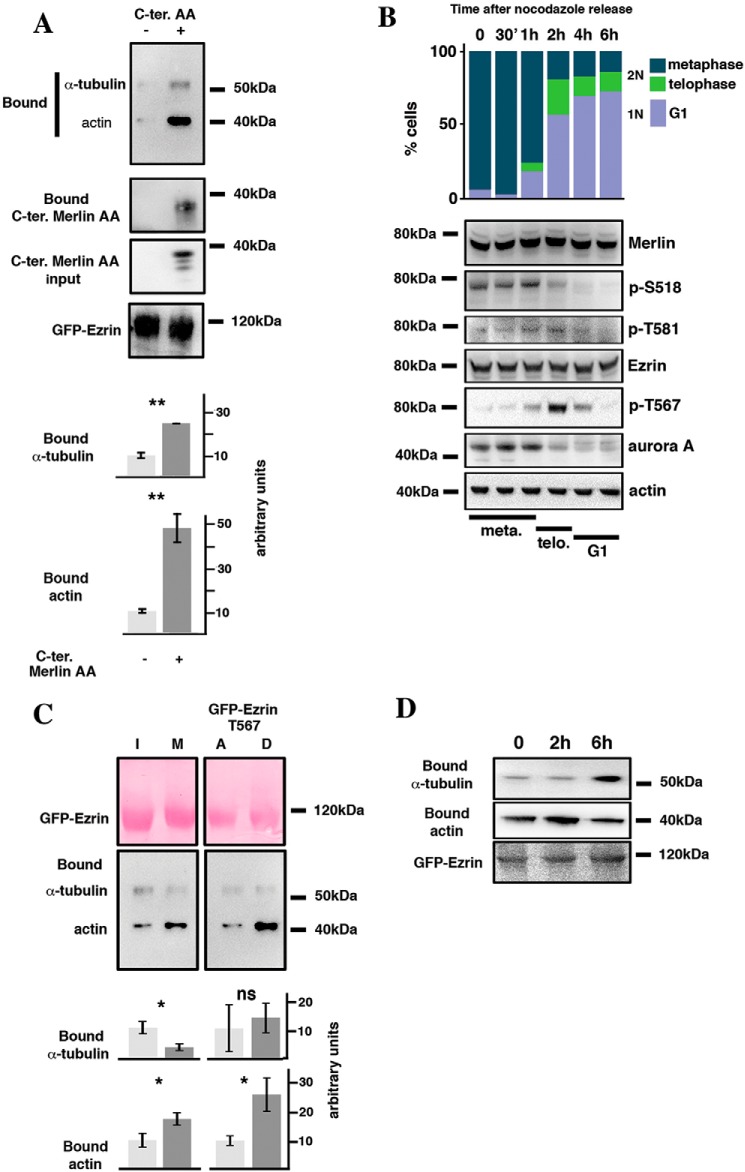
Figure 5.
Merlin and Threonine 567 phosphorylation modulate ezrin interaction with the cytoskeleton during mitosis. A, Merlin C-terminal AA domain binds to ezrin and stimulates its interaction with α-tubulin and actin. Merlin C-terminal AA domain was co-expressed (+) or not (−) with GFP-ezrin in HeLa cells. Bound α-tubulin and actin were evaluated by Western blotting following precipitation of GFP-ezrin. B, the expression and phosphorylation of Merlin (Ser-518 and Thr-581), ezrin (T567), and Aurora A were evaluated by Western blotting following the release of HeLa cells from nocodazole block. Actin was used as loading control. The progression from mitosis (metaphase and telophase, 2N cells) to G1 (1N cells) was followed by FACS and by microscopy using DAPI staining (upper bar graph). The time frame showing the progression of the cells through metaphase and telophase, assessed by DAPI staining, is shown under the blots. C, immunoprecipitation of GFP-ezrin shows that its binding to α-tubulin decreases whereas interaction with actin increases during mitosis compared with interphase. In addition, T567D substitution stimulates actin binding of ezrin but has no impact on α-tubulin interaction, when compared with T567A substitution. A quantification of three independent experiments is provided. Student's t test; ns, p > 0.05; *, p < 0.05; **, p < 0.01; ***, p < 0.001. D, the binding of actin and α-tubulin to GFP-ezrin was followed by co-immunoprecipitation during the transition from mitosis to G1 following release from nocodazole block in HeLa cells.