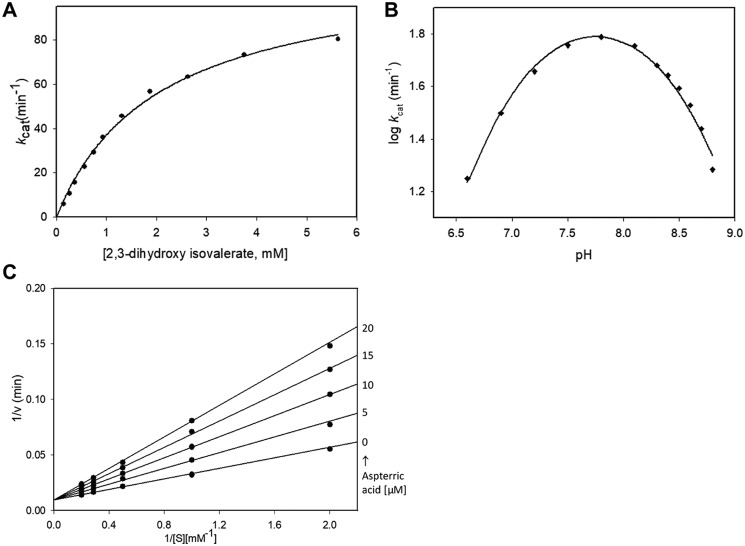
Figure 2.
Catalysis by Mtb-DHAD. A, Michaelis–Menten plot showing activity toward (R)2,3-dihydroxy isovalerate, giving calculated Km and kcat values of 2025 ± 140 μm and 112 ± 4 min−1, respectively. B, pH-activity profile, showing a pH optimum of ∼7.8 and implicating two groups with pKa values of 7.35 ± 0.05 and 8.30 ± 0.04 in catalysis. C, inhibition studies with aspterric acid, conducted over substrate concentrations from 0.5 to 5 mm at fixed inhibitor concentrations up to 20 μm. These plots showed competitive inhibition, with a Ki of 10.1 ± 0.4 μm. The kinetic experiments (A) and pH profile (B) were carried out in duplicate, and the standard errors quoted were obtained by global fitting to single data sets as described under “Experimental procedures.” The inhibition experiments (C) were only carried out once, because of the small quantities of aspterric acid available, with the standard error in Ki obtained by global fitting as described.