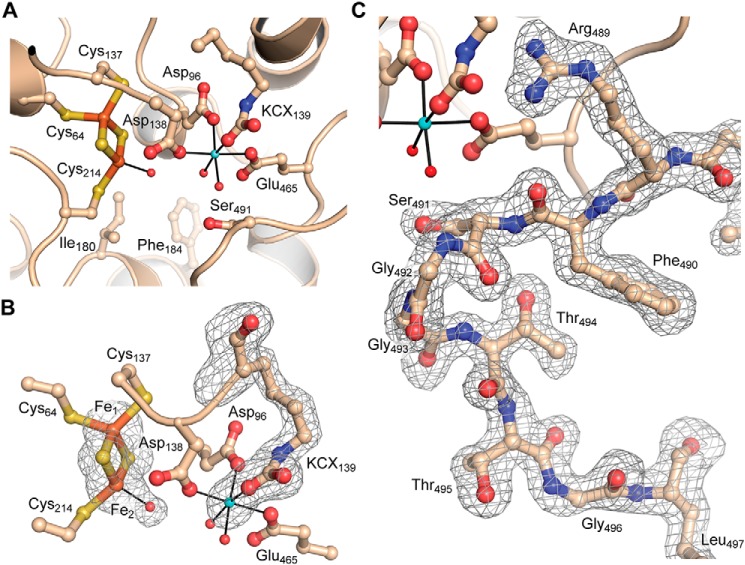
Figure 4.
Active site of Mtb-DHAD. A, overall view of the active site, showing the 2Fe–2S cluster (iron atoms in orange and sulfur in gold), the Mg2+ ion (blue sphere), and the carbamylated lysine residue (KCX139). Water molecules bound to the Mg2+ and to Fe2 of the cluster are shown as small red spheres. The catalytically essential residue Ser-491 can be seen projecting into the active site cavity. Some water molecules in the cavity are omitted for clarity. B, The electron density for the 2Fe–2S cluster and its bound water molecule, the Mg2+ ion, and the carbamylated lysine is shown, from a bias-removed Fo − Fc electron density map calculated in Phenix (28) and contoured at 3 σ. Model bias was removed by omission of the atoms in question, followed by cycles of refinement prior to calculation of the map. C, omit electron density map for the flexible loop that carries the catalytically essential Ser-491. Shown for molecule A and calculated as for B.