Figure 7.
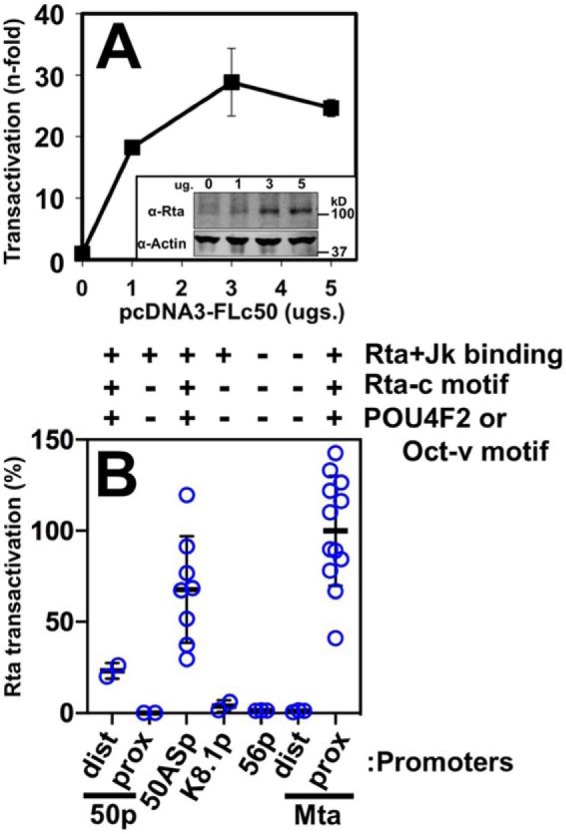
RBP-Jκ DNA binding is not sufficient for Rta transactivation. A, ORF50AS promoter. Reporter plasmid was co-electroporated into BL-41 cells alone or with increasing amounts of Rta expression vector in triplicate. Cells were harvested 48 h post-electroporation, and luciferase was measured. Each luciferase value was normalized to β-gal expression from a second reporter plasmid that was co-electroporated as control. Fold transactivation was calculated by comparison with the promoter reporter transfected alone. Inset, typical results of Western blotting to show expression of ectopic Rta in transactivation experiments. Top panel, Western blotting of total cellular protein probed with anti-Rta serum. Bottom panel, Western blotting from same gel probed with anti-actin. B, other candidate promoters. Each of the indicated reporter plasmids were co-electroporated into BL-41 cells with Rta expression vector following the procedure described in A. The maximal transactivation from each titration curve was normalized to transactivation of the Mta proximal (Mta prox) promoter, which was set at 100%. Thick lines indicate means of values, and thin lines indicate standard errors. Chart at top shows result of Rta + Jk binding from Fig. 2, and the presence or absence of indicated motifs as in Fig. 6. dist prox, distal and proximal.
